a World Health Organization, Port Moresby, Papua New Guinea.
b Pathology Department, Port Moresby General Hospital, Papua New Guinea.
c National Department of Health, Port Moresby, Papua New Guinea.
Correspondence to Manoj Murhekar (e-mail: mmurhekar@gmail.com).
To cite this article:
Murhekar M et al. Vibrio cholerae antimicrobial drug resistance, Papua New Guinea, 2009–2011. Western Pacific Surveillance and Response Journal, 20123, 4(3):60–62. doi:10.5365/wpsar.2013.4.2.002
Cholera is an acute infectious disease caused by Vibrio cholerae. The disease occurs in a variety of forms ranging from sporadic cases to outbreaks that may transition to endemic disease. While cholera case management focuses on early, rapid rehydration, antimicrobial therapy can reduce the volume of diarrhoea, duration of carriage and symptoms and is frequently recommended for patients with severe dehydration.1–4 For this reason, antibiotics are often indicated for the management of moderate and severe cholera case patients. The current World Health Organization and Médecins Sans Frontières guidelines for cholera treatment recommend antibiotics for only severe cases, whereas the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) recommends antibiotics for both severe and moderate cases.5,6
The emergence of antimicrobial drug resistance following the introduction of antibiotics is a commonly reported global phenomenon. Vibrio cholerae remained susceptible to many antibiotics for a sustained period, with only 3% of the isolates demonstrating resistance in the worldwide survey conducted in 1976.7 However, during the past two decades, reports from several cholera-endemic countries of strains resistant to antibiotics including tetracycline, ampicillin, kanamycin, streptomycin, sulphonamides, trimethoprim and gentamicin have appeared.4 Indiscriminate use of antimicrobials is one of the commonest reasons for emergence of resistance.4 For this reason, recommendations for antibiotic use for cholera case management should promote their selective use and be based on the antibiotic susceptibility pattern of Vibrio cholerae in the area.
The first outbreak of Vibrio cholerae O1 biotype El Tor, serotype Ogawa was reported in Morobe province of Papua New Guinea in July 2009.8 Following this outbreak, cholera spread to other provinces and by April 2011, outbreaks were reported in almost half the provinces in the country, causing more than 15 000 reported cases and 493 deaths.9 Occurrence of faecal culture-confirmed cholera diarrhoea in a population for at least three of the past five years is considered as a criteria for defining cholera endemicity in an area.10 As transmission of cholera in Papua New Guinea continues into the third year (2012), the disease would be classified as endemic. During the outbreak, health authorities recommended doxycycline for adults and erythromycin or azithromycin for children and regnant women for the treatment of cases with moderate and severe dehydration.
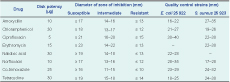
In previously cholera-free districts, health authorities collected stool samples or two rectal swabs from initial cases during outbreaks of acute watery diarrhoea to confirm the etiology. They also collected stool samples sporadically from districts where the outbreaks were ongoing. The stool specimens were sent to the National Reference Laboratory for culture following standard procedures for the isolation and identification of Vibrio cholerae. The stool samples were inoculated on Thiosulphate citrate bile salt sucrose and MacConkey’s agar and incubated at 37˚C for 18–24 hours. The isolated Vibrio cholerae strains were serotyped using polyvalent and monovalent antisera (Denka Seiken Co, Ltd, Tokyo, Japan). Susceptibility to different antibiotics was tested by disk diffusion technique11 following the Clinical and Laboratory Standards Institute (CLSI) guidelines12 using a commercially available disk (Oxoid Ltd, England) of eight antimicrobial agents: amoxycillin (10 μg/disc), chloramphenicol (30 μg/disc), ciprofloxacin (5 μg/disc), erythromycin (15 μg/disc), nalidixic acid (30 μg/disc), norfloxacin (10 μg/disc), co-trimoxazole (25 μg/disc) and tetracycline (30 μg/disc). Standard strains of Escherichia coli ATCC 25 922 and Staphylococcus aureus ATCC 25 923 were used as control strains. Interpretation of zone size was done in accordance with the CLSI guidelines classifying the antimicrobial resistance.12,13 Since there is no Vibrio cholerae-specific CLSI interpretive criteria for several of the drugs for which resistance is described, we considered a zone of inhibition of 21mm for ciprofloxacin, 23mm for erythromycin, 19mm for nalidixic acid and 17mm for norfloxacin as the cut-off values to determine susceptibility (Table 1). We analysed the antimicrobial drug resistance data since the beginning of the cholera outbreak in the country.

During the period August 2009 to April 2011, Vibrio cholerae was isolated from 321 samples, of which 305 (95%) were tested for antibiotic susceptibility. Cholera isolates were of El Tor biotype and Ogawa serotype. Of the 299 isolates tested against tetracycline (proxy for doxycycline), 29 (9.7%) were resistant and 94 (31.4%) showed intermediate resistance. Of the 254 isolates tested against erythromycin, 97 (38.2%) were resistant while 139 (54.7%) demonstrated intermediate resistance. Most isolates (75.8%) were resistant to amoxycillin while the resistance to norfloxacin (0%), nalidixic acid (0.3%), ciprofloxacin (1%) and co-trimoxazole (3.2%) were low (Table 2). A total of 251 isolates were tested for both erythromycin and tetracycline. Of these, 14 (6%) and 60 (24%) showed complete and intermediate resistance to the antibiotics, respectively.
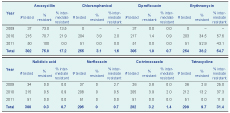
The proportion of isolates showing either complete or intermediate resistance to tetracycline rose from 27.8% (10/36) in 2009 to 50.5% (107/212) in 2010 before decreasing to 11.8% (6/51) in 2011. Isolates were not tested for erythromycin resistance in 2009, but in 2010 and 2011, 92.1% (187/203) and 96.1% (49/51) of the isolates showed intermediate or complete resistance, respectively.
Not all the isolates could be tested for all eight antimicrobials. This was a limitation of the data. We report high levels of resistance to erythromycin among the Papua New Guinea Vibrio cholerae isolates with fluctuating resistance to tetracycline. Health care in Papua New Guinea is delivered through provincial hospitals at provincial level and health centres, rural hospitals and aid posts in the rural areas. The standard treatment guidelines prepared by the National Department of Health are followed in the country for treatment of common ailments in adults and children. Health authorities may consider these susceptibility data when reviewing the national treatment guidelines, as well as the availability, cost, usage and clinical outcomes. While doxycycline may still be considered for the treatment of severely dehydrated cases among adults, an alternative antimicrobial therapy to erythromycin should be considered for pregnant women or children. Monitoring of antimicrobial resistance of Vibrio cholerae should remain a priority for the public health laboratory surveillance system.
None declared.
None.
The authors acknowledge Dr Subarna Roy from the Indian Council of Medical Research for his critical comments on the manuscript.