a Papua New Guinea Institute of Medical Research, Goroka, Papua New Guinea.
b Papua New Guinea National Department of Health, Port Moresby, Papua New Guinea.
c Institut Pasteur de Nouvelle Calédonie, Noumea, Nouvelle-caledonie.
d WHO Collaborating Centre for Arbovirus Reference and Research, Queensland University of Technology, Brisbane, Australia.
e World Health Organization, Port Moresby, Papua New Guinea.
Correspondence to Paul Horwood (e-mail: paul.horwood@pngimr.org.pg).
To cite this article:
Horwood P et al. The threat of chikungunya in Oceania. Western Pacific Surveillance and Response Journal, 2013, 4(2):8–10. doi:10.5365/wpsar.2013.4.2.003
The Oceania region, which includes Australia, New Zealand, Papua New Guinea and the islands of the tropical Pacific Ocean, has historically been free from chikungunya. However, the 2011 outbreak in New Caledonia and the ongoing outbreak in Papua New Guinea have highlighted the risk to other communities in Oceania where there are competent mosquito vectors and permissive social factors and environmental conditions. In this article we discuss the threat to this region that is posed by the recent evolution of the E1:A226V mutant strains of chikungunya virus (CHIKV).
Chikungunya is a mosquito-borne disease caused by infection with CHIKV, an alphavirus from the Togaviridae family. The clinical characteristics of chikungunya include acute onset of fever which may last up to two weeks and painful, potentially debilitating, polyarthritis in adults which may last for up to a year following infection. Chikungunya was first recognized in Africa in the 1950s, principally, as polyarthritis in adults.1 Other symptoms, reported during the large outbreak on Réunion Island in 2005–2006, included maculopapular rash on the trunk and limbs, headache, nausea, vomiting, diarrhoea and fatigue.2
There are three distinct genotypes of CHIKV: (1) Asian, (2) Eastern/Central/Southern African (ECSA), and (3) Western African. The ECSA genotype has been the dominant strain throughout Asia and the islands and countries in the Indian Ocean over the last decade. This genotype gained dominance in 2004 and 2005 when it was introduced from Kenya into the Indian Ocean islands of Comoros, Réunion, Seychelles, Mauritius and Mayotte where it was associated with an outbreak involving hundreds of thousands of reported cases.3 On the island of Réunion, it was estimated that more than 30% of the 770 000 inhabitants were infected by CHIKV.2 In 2005, an epidemic of chikungunya began in India which resulted in more than 1.39 million suspected cases by 2011.4 The ECSA genotype of CHIKV also spread to other Asian countries including Sri Lanka, Malaysia, Singapore, Thailand, Indonesia, China and Myanmar.3,5
Previous outbreaks of CHIKV infection have been associated with the mosquito vector Aedes aegypti, which is also the vector of yellow fever and dengue viruses. However, Aedes albopictus has been the principal mosquito vector during many of the recent outbreaks of chikungunya associated with ECSA strains.6 Analysis of CHIKV from the explosive outbreaks in Réunion and India revealed that the ECSA strains had acquired a point mutation resulting in a change from alanine to valine at position 226 in the E1 glycoprotein which enhanced the transmissibility of CHIKV in Aedes albopictus.7 Subsequent studies demonstrated that amino acid changes in the E2 glycoprotein had a strong modulating effect on the E1:A226V change.8
Until the outbreak of chikungunya in New Caledonia from February to June 2011, which was caused by Asian-lineage CHIKV rather than the E1:A226V ESCA lineage,9 Oceania had been free from chikungunya. During this outbreak, only 33 cases were detected, attributed to the onset of the cold season and the comprehensive control measures implemented after the diagnosis of the first cases.
In June 2012, an outbreak of fever and arthritis was detected in Vanimo, Papua New Guinea. Subsequent investigations showed that the outbreak was caused by an ECSA strain of CHIKV which harboured the E1:A226V mutation. During the Vanimo outbreak more than 1500 suspected cases of chikungunya were reported through passive surveillance.10 The vector in this outbreak was suspected to be Aedes albopictus due to the high density of this mosquito species in the area. Following this outbreak, chikungunya cases were confirmed by real-time reverse-transcriptase polymerase chain reaction from eight provinces of Papua New Guinea, with another three provinces having suspected outbreaks. Interestingly, the outbreak extended to the Highlands Region of Papua New Guinea, which is the first confirmed arboviral outbreak recorded in this region of the country. Although no entomologic surveys have been conducted in the Highlands Region for many years, it has been shown that Aedes mosquitoes are present in abundant numbers. This may have important implications as more than 50% of the Papua New Guinea population live in the Highlands Region.
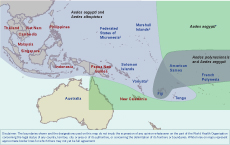
In Oceania, a considerable number of endemic mosquitoes belonging to the genus Stegomyia and the “Scutellaris group,” such as ,Aedes polynesiensis in French Polynesia, are recognized as vectors of CHIKV.11,12 More importantly, the principal vectors of chikungunya, Aedes aegypti and Aedes albopictus, are both prevalent throughout the region. Aedes aegypti is present in all countries in the Pacific except for New Zealand, Futuna and some small isolated islands.13 Aedes albopictus invaded Oceania in the 1960s14 and now can be found throughout Papua New Guinea, the Torres Strait region of Australia, Fiji, Solomon Islands, Tonga and probably Vanuatu,13,15 thus rendering the human populations of these islands vulnerable to introduction of the epidemic ECSA strains of CHIKV. The explosive outbreak of chikungunya in the Indian Ocean islands and the speed with which the related alphavirus, Ross River virus, swept through the Pacific in 1979 and 1980,16 is a reminder of the potential impact CHIKV could have in Oceania (Figure 1).

Note: Countries in red had previous chikungunya outbreaks.
* Aedes aegypti is found throughout the region except in Futuna and some isolated islands.
† The presence of Aedes albopictus has not been officially confirmed in Vanuatu; however, its presence is strongly suspected.
‡ Aedes albopictus has not been detected in the Marshall Islands or the Federated States of Micronesia; however, its presence is suspected due to the proximity of islands such as Guam and Palau where the vector has been confirmed.
Social, economic and environmental factors all play an important role in the introduction and sustained transmission of arboviral diseases like chikungunya. In developing countries such as Papua New Guinea and many other Pacific island communities, poor living conditions and the abundance of natural and artificial mosquito breeding sites can result in the rapid spread of arboviral epidemics. The climatic conditions of Oceania (temperature, humidity) favour year-round mosquito breeding and are unlikely to interrupt the transmission cycle of CHIKV. It is doubtful that any Pacific island community has the human or financial resources to mount a vector control effort that would prevent an outbreak of chikungunya. However, efficient surveillance, targeted vector control (including active community participation for breeding sites elimination) and education in mosquito avoidance measures may provide a cost effective reduction in the burden of disease in the event of an outbreak. A coordinated regional strategy to prevent and respond to vectorborne disease outbreaks in Oceania is urgently needed to mitigate future outbreaks of arboviral diseases such as chikungunya and dengue.
None declared.
None.
Conflicts of interest
Funding
References:
and laboratory features in 157 adult patients. Clinical Infectious Diseases, 2007,
44:1401–1407.
doi:10.1086/517537
pmid:17479933
5:33–36.
doi:10.1186/1743-422X-5-33
pmid:18304328
Papua New Guinea. Emerging Infectious Diseases, 2013
doi:10.3201/eid1909.130130