a School of Health Science, Pacific Adventist University, Atoifi Campus, Atoifi, Malaita, Solomon Islands.
b School of Medical and Applied Sciences, Central Queensland University, North Rockhampton, Australia.
c Community Leader, Na’au, East Kwaio, Malaita, Solomon Islands.
d Atoifi Adventist Hospital, Atoifi, Malaita, Solomon Islands.
e Community Leader, Gounaasuu, East Kwaio, Malaita, Solomon Islands.
f Community Leader, Ambitona, East Kwaio, Malaita, Solomon Islands.
g Community Leader, Batuna, New Georgia, Western Province, Solomon Islands (deceased).
h Community Leader, Wyfolonga, East Kwaio, Malaita, Solomon Islands.
i Community Leader, Sifilo, East Kwaio, Malaita, Solomon Islands.
j Department of Pharmacy and Applied Science, La Trobe University, Bendigo, Australia.
k College of Medicine and Dentistry, James Cook University, Cairns, Australia.
l Hunter New England Population Health, Tamworth, Australia.
m Tropical Health Solutions, Townsville, Australia.
n College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, Australia.
Correspondence to Rick Speare (email: rickspeare@gmail.com).
To cite this article:
Harrington H et al. Prevalence of soil-transmitted helminths in remote villages in East Kwaio, Solomon Islands. Western Pacific Surveillance and Response Journal, 2015, 6(3). doi:10.5365/wpsar.2015.6.1.016
Objective: Although soil-transmitted helminths (STH) are endemic in Solomon Islands, there are few recent reports on their prevalence. This study aimed to determine the prevalence of STH in residents of remote communities in Solomon Islands.
Methods: A cross-sectional convenience-sampled survey of residents of four adjacent villages in Malaita, Solomon Islands was performed in Atoifi and Na’au in April 2011 and in Abitona and Sifilo in April 2012. All residents older than one year were invited to participate, which involved providing a single sample of faeces examined using a modified Kato-Katz technique and completing a questionnaire that asked demographic and STH-related behaviour questions.
Results: The overall participation rate was 52.8%, with 402 participants comprising 49.8% males. Hookworm was the predominant STH with only a single case of trichuriasis found in Atoifi. The total prevalence of hookworm was 22.6% (95% confidence interval: 18.6–27.1); the prevalence of hookworm in Abitona, Na’au and Sifilo was 20.0%, 29.9% and 27.4%, respectively, whereas in Atoifi it was 2.3% (P < 0.001). Intensity was low in all villages. Although health behaviours differed significantly between Atoifi and the other three villages, the type of toilet used was the only significant association with hookworm.
Discussion: Residents of Atoifi have a relative freedom from STH compared to the other three villages. Rather than a region-wide morbidity control approach, a “one village at a time” approach aiming to eliminate STH and dealing with each village as a separate autonomous unit empowered to manage its own challenges may be a preferred option.
Soil-transmitted helminths (STH) are endemic in Pacific island countries and territories, yet there is little recent published data on country-specific prevalence.1,2 STH include a small number of parasitic intestinal nematodes; the major species are roundworm (Ascaris lumbricoides), hookworm (Ancylostoma duodenale, Ancylostoma ceylanicum, Necator americanus), whipworm (Trichuris trichiura) and Strongyloides stercoralis. STH are a significant cause of morbidity in vulnerable groups such as children and pregnant women. A World Health Assembly resolution required that, by year 2010, regular treatment at appropriate intervals be offered to 75–100% of all school-age children living where STH have public health consequences.3 The current World Health Organization (WHO) approach focuses on STH morbidity control, using anthelmintics combined with health education, to target primary schoolchildren and pregnant women.3–5 However, this will not prevent transmission and has been criticized for not emphasizing provision of appropriate sanitation and promotion of behaviours to reduce STH transmission.6
Individual communities may wish to eliminate STH rather than reduce morbidity, and for some communities large-scale government or external donor-driven programmes may not be the preferred model.7 Small isolated villages, in remote areas may also be missed in national programmes due to logistic difficulties. Hence, in remote areas with isolated villages, a programme driven from the village level may be more acceptable, cost effective and sustainable.
Solomon Islands is a tropical country in the Pacific and is ranked 157/186 on the United Nations Development Programme Human Development Index.8 STH are prevalent in Solomon Islands, although little information has been published.1 A recent review listed Solomon Islands as having the second highest number of cases of trichuriasis in Oceania and the third highest number for hookworm and ascariasis.2 Results from only two faecal surveys are available: a survey in two primary schools in Honiara, the nation’s capital, in 2001–2002 found prevalence of STH of 41–45% with A. lumbricoides (2.5–3.4%), hookworm (25–32%) and T. trichiura (17–25%).9 A recent survey of 295 children found the prevalence of STH was 81% with prevalences of hookworm (58%), T. trichiura (24%), A. lumbricoides (33%) and S. stercoralis (16%).10 Prior to the latest survey of schoolchildren, two studies reported that foreign personnel of the Regional Assistance Mission to Solomon Islands had acquired S. stercoralis even though this STH had not been reported in Solomon Islands residents at that time.11,12 Solomon Islands currently is not meeting its target of treating 75% of primary school-age children.13
Building in-country capacity in the surveillance and research of STH has been proposed as essential to sustainable and committed response programmes.14 This study was used to build capacity in health professionals at Atoifi Adventist Hospital (AAH)15–17 and local community members in East Kwaio, Malaita to conduct surveys for STH. A key principle adopted is that the community determines the questions to answer by their research.17,18 The aims of this study were to determine the initial prevalence and intensity of STH in residents of four villages in East Kwaio and to relate these to health behaviours in order to guide locally-determined interventions. These surveys will be repeated to assess the effectiveness of the response programmes.
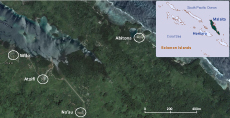
The AAH is located in East Malaita and provides health care to the population of East Kwaio (Figure 1). All hospital staff and nursing students of the Atoifi College of Nursing live in Atoifi, a village surrounding the AAH. There is no road access to Atoifi and people travel by light aircraft, boat, canoe or foot. AAH constructs and maintains its own housing, electricity supply, water, sewage and communication systems. Atoifi has a total population of 214 housed in permanent Western style buildings with flush septic toilets inside the houses. Nearly all residents are Solomon Islanders. At least one member of each family is employed by the hospital and receives a regular salary with average annual cash income per resident being about US$ 7.50 per day.

Source: Inset map of Solomon Islands was generated using WHO HealthMapper and the detailed map of the four villages of East Kwaio was from Google Maps
(https://www.google.com.ph/maps/place/Malaita+Province,+Solomon+Islands/
@-8.8624862,160.9825196,7760m/data=!3m1!1e3!4m2!3m1!1s0x6f25eeb2f8a1cf2d:
0xbe7b57968407b8a7!6m1!1e1).
Abitona, Na’au and Sifilo are typical Solomon Islands rural villages within five kilometres of Atoifi (Figure 1). Abitona and Sifilo are on the coast, while Na’au is about one kilometre inland situated beside a river. Houses are permanent or semi-permanent and made of a combination of traditional and Western building materials. In these three villages there were only two formal toilets: in Na’au one house had a pit latrine situated outside the house, and in Abitona a water-seal toilet was available at the village guest house. The villages had separate environmental toileting areas for men, women and children. In all three villages residents defaecated in the bush, and for Abitona and Sifilo it was also done in the sea or mangroves. Na’au had separate community toilets for men and women that each consisted of a plank on the edge of a natural depression about 5–10 minutes’ walk from the village centre. Most residents of these villages rely on subsistence farming, selling agricultural and other produce, or remittances from family members working in other locations. The average annual cash income per resident was observed to be less than US$ 2 per day.
Villages in East Kwaio are characterized by densely populated villages of 50–200 residents separated by thick rainforest, cocoa and coconut plantations or periodic slash-and-burn gardens with no dwellings.
Residents of the four villages move freely between these and similar local villages for social or religious activities. Residents of Abitona, Na’au and Sifilo also enter Atoifi to access medical and health services, to purchase goods or to access bank and travel services. Residents of Atoifi also enter Abitona, Na’au and Sifilo to deliver health outreach or to investigate outbreaks.
This cross-sectional faecal survey for STH also included a questionnaire which asked the age and sex of participants as well as four questions relevant to STH transmission (Table 1). The questionnaire was self-administered for literate participants; for participants with low literacy it was completed by parents for their children or through interviews by local researchers. All members of the four communities, excluding children less than one year of age, were invited to participate through word of mouth. Surveys were conducted for Atoifi and Na’au in April 2011 and Abitona and Sifilo in April 2012. All participants were assigned a unique identification code to preserve anonymity with the key to the codes retained by the lead author.
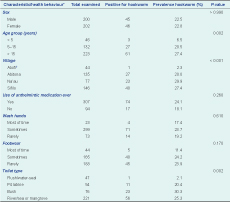
* Some variables may not add up to the total (n=402) due to missing values.
† Kruskal–Wallis test.
CI, confidence interval.
Faeces were examined within 12 hours of excretion at the AAH laboratory. A modified Kato-Katz technique using a 41.7 mg mould was used, resulting in a multiplication factor of 24 to calculate eggs per gram (EPG).19 Modifications made to the standard technique consisted of adding an equal volume of normal saline and covering the faecal mix with a 24 mm x 40 mm glass coverslip instead of cellophane soaked in glycerol and malachite green.20 This method eliminates the problem of the rapid clearing of hookworm eggs by glycerol, which may reduce the detection of hookworm eggs by up to 50%.21 Intensity of infection determined from EPG was classified into light, medium and heavy using WHO criteria.22
Data were entered into an Excel file and statistical analyses were performed using SPSS Version 22. Categorical variables were expressed as percentages; exact binomial confidence intervals were calculated. Numerical variables were expressed as means and standard deviations or medians (interquartile range) when normality assumptions were not fulfilled. Bivariate tests between two categorical variables were conducted using exact binomial tests (trend test versions where applicable and noted). The Kruskal–Wallis test was used to compare ages across villages.
Ethical approval for the study was obtained from James Cook University Human Research Ethics Committee (H4002) and the AAH Research Ethics Committee (AAHREC1). All participants or their guardians (for children) signed a consent form and were given individual treatment with albendazole if STH were found in their faeces.
Samples and questionnaires were provided by 402 people, giving an overall participation rate of 52.8% (402/761). The 96 residents who failed to provide both a faecal sample and a completed questionnaire were excluded. Males constituted 49.8% (200/402); the age of participants ranged from 1 to 90 years, with 11.5%, 32.9% and 55.6% in the age groups of children under 5 years, 5–15 years and over 15 years, respectively.
The overall prevalence of hookworm was 22.6% (91/402). Hookworm was found in 22.5% of males (45/200) and 22.8% of females (46/202) with no association by sex (P = 0.86). Hookworm prevalence increased significantly with age (P = 0.002; Table 1). There was no significant association between the prevalence of hookworm and the use of anthelmintic medication, hand washing or the use of footwear. However, the type of toilet used was significantly associated with hookworm (P = 0.002; Table 1).
Participation rates by village ranged from 20.6% for Atoifi to 93.1% for Abitona. The mean age of participants from each village was 25.6 years for Abitona, 21.3 years for Atoifi, 30.1 years for Na’au and 20.9 years for Sifilo (Table 2).
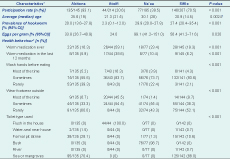
* Some variables may not add up to the total (n=402) due to missing values.
† Kruskal–Wallis test.
CI, confidence interval.
The prevalence of hookworm in Atoifi was 2.3%, yet it was 20% or greater for the other three villages; this was significantly different (P < 0.001; Tables 1 and 2). Only hookworms were found in Abitona, Na’au and Sifilo, whereas Atoifi also had one case of T. trichiura. All cases of hookworm infection were in the light category with < 2000 EPG (Table 2).
Health behaviours differed significantly among villages (Table 2). All participants from Atoifi used a flush toilet inside the house, while nearly all from Na’au used the bush. For the two villages on the shore, Abitona and Sifilo, participants mainly used either a pit latrine (personal observation revealed these were natural holes in the rocky slopes) or the sea/mangroves.
Within two weeks of the survey, individuals diagnosed with STH were offered albendazole at standard dose rates. Within a month of the survey the residents in Abitona, Na’au and Sifilo participated in a village-wide mass drug administration with albendazole provided by AAH. Coverage was 100%. Within three months residents of Na’au also initiated a project to drain standing water around the village and to build gravel walkways to reduce contact with damp soil, a novel intervention due in part to the findings of the STH survey.
Previous surveys for STH in primary schoolchildren from urban areas of Solomon Islands found the prevalence of hookworm to be 25–58%.9,10 The prevalence in this study from the three remote villages, Abitona, Na’au and Sifilo, were at the lower end of this range at 20.0% to 29.9%. We found only hookworm and T. trichuria, but in earlier surveys Ascaris and S. stercoralis were also detected.9–12 The prevalence of hookworm increased with the age group in this study, a trend similar to that seen in Tuvalu, another small Pacific island nation.23
The prevalence of STH in Atoifi was low, just a fifth of that found at the three adjacent villages. There has been only one similar report of an individual village with a low prevalence of soil-transmitted intestinal parasites (helminths and protozoa) in a highly endemic region: 4.5% versus 73% in Sungai Layau village in West Malaysia.24 This village had better housing and residents used the amenities.24
Abitona, Na’au and Sifilo differed from Atoifi as they had no formal toilets and practise environmental defaecation. Although over a quarter of Abitona residents reported using pit latrines, these were actually deep natural holes in the rocky slopes and not formal toilets. This may explain the differences in STH prevalence as improved sanitation has been shown to be protective against hookworm.25 Although behaviours likely to reduce transmission (e.g. use of footwear, frequent hand washing) and to decrease prevalence of STH (use of anthelmintics) were less commonly practised by residents of the three villages when compared to Atoifi, these were not significantly associated with hookworm prevalence.
An elimination strategy for neglected tropical diseases, of which STH are a category, has been advocated at the global level.26 Owing to logistic difficulties and the cost of bringing outside groups in to implement control programmes, the small isolated villages in our survey may be missed in national programmes, but a programme that is driven from the village level with local health professional support may be more cost effective, sustainable and responsive to local needs. For individual villages, the epidemiology of their STH can be determined and linked with social mapping to enable village-specific risk factors to be identified.27 There is an opportunity to move from morbidity control to elimination of STH at a village level in Solomon Islands.
We recommend that a STH elimination programme be tailored to each village using a capacity-building model. This should train local health professionals to conduct STH surveys and recruit communities through health education and detailed discussions on how STH can be eliminated from their villages with agreement that the community will work to improve their sanitation. The focus of control efforts in East Kwaio should be an integrated approach that includes safer defaecation, improved hand-washing, and use of footwear, particularly in villages where hookworm is found, combined with anthelmintic therapy.6,28
This “one village at a time” approach is needed due to the widespread failure of previous region-wide sanitation projects in this area of Malaita where toilet hardware had arrived from overseas donors with no resident involvement. In the area of this study, for example, the only evidence of a regional programme a decade ago to provide toilet hardware was a single toilet in Abitona adjacent to the village guest house. Similarly, an evaluation of a programme in Vanuatu that distributed VIP-toilets at a regional level found they were not used for various reasons and proposed a model of targeting a small number of communities at a time.29 As the villages in East Kwaio are isolated and separated by areas of low human habitation such as forests or gardens, we hypothesize that STH are largely acquired in a resident’s home village, providing further support for the “one village at a time” approach. In a similar approach to eliminate yaws in rural Solomon Islands the village was proposed as the most effective unit rather than the family or region.30 Acquisition of STH outside a resident’s home village is possible, particularly from STH hot spots where eggs or larvae in faeces on the soil have grown to infective stages.31–33 Hence, occasional low-level STH infection should be expected from outside the home village.
Choosing toilets for these communities is a complex issue determined by physical, social, cultural, technical and economic factors that vary from village to village and even within the same village. For example, some Abitona and Sifilo residents use the sea for defaecation; others, who live on the slopes, use natural holes in the ground and the bush. An example of the cultural complexities occurs in the largely Christian village of Na’au, located on the foot road into the East Kwaio mountains. Most people who live in the East Kwaio mountains are very traditional and practise ancestor worship.34 The leaders of Na’au respect the traditional beliefs and acknowledge the importance of gender by making provision for single-sex toilets located in male and female areas as well as shared toilets for Christian households located separately.35
Although this study was not large for a STH survey in terms of absolute numbers and involved convenience samples from residents of four adjacent villages, a substantial proportion of the available population was recruited. The use of a single faecal sample will underestimate the prevalence of STH.36 The lack of funds and time and difficulties for village residents to provide samples when using open forest or coastal areas for defaecation limited the survey to a single sample per participant. The modified Kato-Katz technique may have also underestimated the prevalence and intensity since clearing of the faecal matter does not occur. Since the two surveys were not done contemporaneously, the 12-month separation may have impacted results. However, since no deliberate STH intervention occurred in Abitona and Sifilo, pre-survey results and results between surveys were similar for Na’au and the other two villages; an effect due to the 12-month delay seems unlikely. Although no S. stercoralis was found, examination using direct smear techniques are less sensitive than the specialized agar plate technique.37,38 As with any cross-sectional survey this study is unable to determine causation.
For rural areas of Solomon Islands we propose that a “one village at a time” approach could be used to eliminate STH from individual villages in regions with small, densely populated villages separated by areas with low human habitation such as forests or gardens. Rather than a region-wide morbidity control approach, a “one village at a time” approach aiming to eliminate STH, and dealing with each village as a separate autonomous unit that is empowered to manage its own challenges may be the option preferred by the residents.
None declared.
This study received financial support from TDR, the Special Programme for Research and Training in Tropical Diseases, cosponsored by United Nations Children’s Fund, United Nations Development Programme, the World Bank and WHO (grant 1–811001688); from the Australian Institute of Tropical Health and Medicine Development Grant; and from Tropical Health Solutions.
Thanks to Robert Strachan for preparation of Figure 1 and to Atoifi Adventist Hospital for accommodation and access to facilities.