a Atoifi Adventist Hospital, Atoifi, Malaita, Solomon Islands.
b Health Protection, Hunter New England Population Health, Tamworth, Australia.
c Tropical Health Solutions Pty Ltd, Townsville, Australia.
d College of Medicine and Dentistry, James Cook University, Cairns, Australia.
e College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, Australia.
Correspondence to Rick Speare (e-mail: rickspeare@gmail.com).
To cite this article:
Oloifana-Polosovai H et al. A marked decline in the incidence of malaria in a remote region of Malaita, Solomon Islands, 2008 to 2013. Western Pacific Surveillance and Response Journal, 2014, 5(3). doi:10.5365/wpsar.2014.5.3.002
Setting: Atoifi Adventist Hospital (AAH), Solomon Islands, the only hospital in the East Kwaio region.
Objective: To use routine surveillance data to assess the trends in malaria from 2008 to 2013.
Design: Descriptive study of records from (1) AAH laboratory malaria records; (2) admissions to AAH for malaria; and (3) malaria treatments from outpatient records.
Results: AAH examined 35 608 blood films and diagnosed malaria in 4443 samples comprised of 2667 Plasmodium falciparum (Pf) and 1776 Plasmodium vivax (Pv). Between 2008 and 2013 the total number of malaria cases detected annually decreased by 86.5%, Pf by 96.7% and Pv by 65.3%. The ratio of Pf to Pv reversed in 2010 from 2.06 in 2008 to 0.19 in 2013. For 2013, Pf showed a seasonal pattern with no cases diagnosed in four months. From 2008 to 2013 admissions in AAH for malaria declined by 90.8%, and malaria mortality fell from 54 per 100 000 to zero. The annual parasite index (API) for 2008 and 2013 was 195 and 24, respectively. Village API has identified a group of villages with higher malaria incidence rates.
Conclusion: The decline in malaria cases in the AAH catchment area has been spectacular, particularly for Pf. This was supported by three sources of hospital surveillance data (laboratory, admissions and treatment records). The decline was associated with the use of artemisinin-based combined therapy and improved vertical social capital between the AAH and the local communities. Calculating village-specific API has highlighted which villages need to be targeted by the AAH malaria control team.
In the 22 Pacific island countries and areas, malaria is endemic in Papua New Guinea, Solomon Islands and Vanuatu.1 Malaria is close to elimination in Vanuatu only in Tafea Province and in Solomon Islands only in Isabel Province. Solomon Islands has been successful in reducing malaria incidence and mortality. The annual parasite index (API), was reduced from 82 in 2008 to 45 in 2012.2 Mortality was 15.7, 7 and 3 per 100 000 in 2003, 2007 and 2012, respectively.2,3 In Malaita Province, the API was 137, 83 and 33.5 in 1996, 2009 and 2011, respectively.4–6 Apart from reducing malaria cases and deaths, the Ministry of Health has the goal of eliminating malaria from two of nine provinces: Isabel and Temotu. The recent and ongoing outbreak of dengue in Solomon Islands has highlighted the challenges of vector control and health education relevant for malaria.7
A national policy for all patients to receive a diagnostic malaria test if suspected was implemented in 1968.8 In the mid-1970s, the API was 30 with malaria largely controlled by residual dichlorodiphenyltrichloroethane (DDT) sprayed inside houses.9 With the cessation of DDT use in 1993 the API rose to 400.10 Insecticide treated nets (ITNs) were trialled in the early 1990s, and national distribution began in 1996.8,10 In 2008 artemisinin-based combination therapy (ACT) was made free for all ages.8 The reported malaria cases (confirmed) in Solomon Islands fell more than 50% between 2000 and 2009 from 368 913 (68 107) to 84 078 (33 002) cases.11,12 In the Atoifi Adventist Hospital (AAH) catchment area, the setting for this study, ITNs were made available in the 1990s but not distributed to all residents. New ITNs were distributed in November 2010 but to five villages only. In July 2009, ACT became the only malaria treatment available in the AAH outpatients department (OPD).
A laboratory scientist at the AAH laboratory noted a decline in the number of malaria cases and the proportion of cases due to Plasmoduim falciparum (Pf) between 2008 and 2013. However, the laboratory data had not been analysed. The aims of this study were to use the data from malaria tests performed at AAH from 2008 to 2013 to (1) describe the trend in confirmed malaria and the proportion of Pf to Plasmodium vivax (Pv); (2) confirm any trends in the laboratory data by assessment of malaria treatment and admission data; and (3) determine the API for the AAH catchment area and for major villages in this area for 2008 and 2013. The Regional Action Plan for the Control and Elimination of Malaria in the Western Pacific (RAP) calls for strengthening national routine surveillance systems to monitor malaria trends and programme impact (Objective 6).1 Hence, this project aligned well with the RAP.
Descriptive study involving review of (1) laboratory records of malaria tests; (2) admissions for malaria; and (3) prescription of malaria treatments.
Lying between latitudes 6° and 12° South, Solomon Islands is a tropical country with a total population of 515 870 (2009 census) and is ranked 143 out of 187 on the Human Development Index.13,14 AAH is in Malaita Province, located on the remote east coast of the island of Malaita (population 137 597) at Uru Harbour and services the people of the East Kwaio region. There is no road access to Atoifi. People travel by light aircraft, boat, canoe or foot. AAH is in a unique situation of being the only hospital and centre for malaria microscopy in East Kwaio, and it is also the only malaria treatment centrea in the Uru Harbour area. AAH provides primary health care services and inpatient services for patients referred from distant clinics.
Testing criteria: any patient presenting or referred to OPD with a fever has a blood sample taken for a malaria film before administration of any treatment. This is standard practice consistent with the national policy.8 A malaria microscopist is always available and examines the film soon after collection or within 48 hours.
The following details are recorded for every malaria test: date of test, patient name, age, sex, village and result. For this study, all specimens were thick blood films stained with Romanovsky stain (Chem-Supply Pty., Ltd, Australia). Specimens were microscopically examined under oil immersion; and results recorded as malaria species: Pf, Pv or mixed (Pf and Pv). For calculations, mixed infections were counted as Pf. All results were written in a malaria laboratory book. Examinations were done mainly by the same two scientists; when they were away, another three laboratory technicians provided results. Rapid diagnostic tests are available at AAH, but so rarely are they done that no data was available.
For this study each laboratory record from 2008 to 2013 was entered into Microsoft Excel (2010) and analysed.
For a 10-week period in 2009, from 23 July 2009 to 30 September 2009, records were missing from laboratory books and could not be found. An estimate was generated for total tests and positive cases by averaging data for the missing period using matching values for 2007, 2008 and 2010.
Admissions to the hospital for malaria were obtained from the AAH admission register. The case definition was based on the Solomon Islands Health Information System, whereby that a patient with clinical malaria had symptoms of malaria (confirmed), was hospitalized and was treated with antimalarial drugs. Details collected included age, sex, outcome and length of stay.
The use of antimalarial drugs over the same period was accessed from the records of AAH OPD. Since malaria treatment for inpatients is always initiated in OPD, these records also capture inpatient treatments.
People who access services at AAH are primarily from two administrative districts, Gulalofou (Ward 17) and Waneagu/Taelanasina (Ward 18). From the 2009 census, the populations of these districts were 6031 and 3478, respectively, totalling 9509.13 This figure was used as the denominator for calculating API, annual blood examination rate (ABER), malaria admission rate and malaria mortality rate for the AAH catchment area. API is the number of confirmed malaria cases per 1000 population per year, and ABER is the number of tests done for malaria per year, expressed as a percentage of the catchment population. Solomon Islands has an annual 2.3% population growth.13 For Malaita Province the annual population growth rate was 2.51%. Using 2009 as the starting point, the population was estimated for the other years using the more conservative 2.3% annual increase.
Over 150 villages are in the AAH catchment area with the largest having approximately 300 residents. To provide a denominator to calculate API and ABER at the village level, Family Heath Cards were used. To obtain data to complete these cards, AAH conducts a regular village demographic survey for the Ministry of Health. Data were available for 2008 but not for 2013. Using a conservative approach, the 2008 population numbers were used as the denominator in 2013. API and ABER for villages were calculated by dividing the number of malaria cases and blood films examined by the population and multiplying by 1000.
Rates of malaria were calculated for the two-year period 2008–2009, prior to and during the introduction of the medication CoArtem® (Novartis, USA) and compared to the most recent two-year period 2012–2013. Two-year periods were used to increase the strength of the comparison. The rate ratio between periods was calculated with a 95% confidence interval (CI).
Results for malaria control in the AAH catchment area were then reviewed in line with the criteria in the RAP.1
Ethics approval was given by the Atoifi Human Research Ethics Committee.
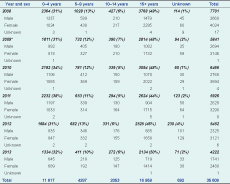
From 2008 to 2013 (missing 10 weeks of data in 2009), the AAH examined 35 608 blood films for malaria (Table 1); the number of cases tested decreased 45.2% over this period.

* The data for 2009 are incomplete as 10 weeks of laboratory records were unavailable.
Males accounted for 44% (15 816/35 571) of the tests and children under five years for 33% (11 617/34 916) (Table 1). The proportion of tests by sex and age group remained similar over the period.
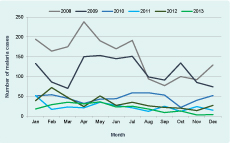
The number of cases of malaria and percent positive were highest in 2008 (1817 and 23.6%) and lowest in 2013 (246 and 5.8%) (Figure 1 and Table 2). Between 2008 and 2013 the total number of positive cases of malaria decreased by 86.5%, Pf by 96.7% and Pv by 65.3%. The ratio of Pf to Pv reversed in 2010 from 2.059 in 2008 to 0.194 in 2013 (Table 2). The change in ratio was greatest between 2012 and 2013.

Pf, Plasmodium falciparum; Pv, Plasmodium vivax.
* The data for 2009 were incomplete as 10 weeks of laboratory records were unavailable.
Fourteen mixed infections of Pf and Pv were diagnosed and counted as Pf. In 2012, cases of malaria, Pf and Pv rose, but this was reversed for Pf in 2013 with a 78.8% decrease in cases, while Pv had a decrease of 4.6%.
For the period 2008–2009, the annual malaria incidence rate was 13 906 per 100 000 population; for the period 2012–2013, the rate was 3161 per 100 000. The rate ratio was 0.23 (95% CI: 0.21–0.25), showing a significant reduction.
Malaria was diagnosed in all months for Pv from 2008 to 2013 and for Pf from 2008 to 2012. In 2013, Pf was not diagnosed in four months; the pattern of Pf changed from a year-long transmission to a suggestion of a seasonal pattern with transmission mainly in the first five months of the year (Figure 2).
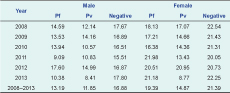
The mean age of malaria cases was lower for both males and females than negative cases (Table 3). The mean age of Pv cases was less than that of Pf cases for both sexes for most years. The difference in mean age was greatest in 2013 (Table 3).
Pf, Plasmodium falcifarum; Pv, Plasmodium vivax.
Hospital admission records for malaria were available for all six years except for the first three months of 2008 (Table 4). Males and children under five years made up 50.3% and 43.3% of total admissions, respectively. The number of admissions declined 90.8% from 2008 to 2013, and the number of deaths fell to zero from 54 per 100 000 in 2008 (Table 4). Length of stay did not change. Hospital policy from 2009 was to keep all malaria inpatients until the course of ACT was complete.
* Missing admission records for January to March for 2008.
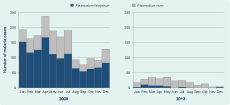
Over the six-year period, data were missing for 10 of the 72 months (Table 5). The number of malaria treatments fell 91% from 2008 to 2013 (Table 5). Chloroquine, Fansidar and quinine were not used after 2009. The category of “no specific details” contains records that stated malaria treatment was given, but no drug details were recorded in OPD records. After July 2009, this category could include only ACT as all other malaria treatments were removed from the AAH OPD.

ACT, artemisinin-based combination therapy.
* Missing data for January to February 2008.
† Missing data for October to December 2009.
‡ Missing data for January to February 2010.
§ Missing data for April to June 2013.
The API for the Atoifi catchment area declined from 195 in 2008 to 24 in 2013, while ABER halved from 83% to 40% for 2008 to 2013, respectively (Table 6).
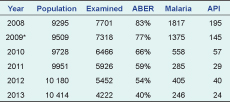
ABER, annual blood examination rate; API, annual parasite index.
* 2009 has estimates for missing data.
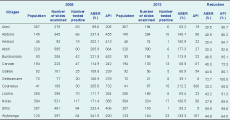
For villages with a reliable 2008 population estimate, the highest API of 732 was for Gounasuu (Table 7). Gounasuu also had the highest API in 2013 although it had declined by 50% to 366.
This study reports confirmed malaria cases over six years (2008 to 2013) from a major hospital in a remote region of Solomon Islands. Since every febrile case or suspected malaria case has a blood test, positive cases of malaria were always laboratory-confirmed cases. This contrasts with presumptive clinical reporting of malaria cases which overestimates malaria incidence.15 In our study, a presumptive clinical diagnosis of malaria would have overestimated incidence by approximately four times in 2008 and 17 times in 2013. The other unique aspect is that AAH is the only source for malaria treatment in the Uru Harbour area. AAH therefore captures a high proportion of symptomatic malaria cases in the Uru Harbour region. The AAH data provide a reliable estimate of the incidence of malaria in the AAH catchment area.
A large and significant reduction in the rates of diagnosed malaria occurred during the period 2008 to 2013. The data show a remarkable fall in the number of blood films positive for malaria, particularly for Pf. The fall in the number of Pf cases was so great that in 2013, no cases were diagnosed in four months and a pattern consistent with seasonal transmission appeared for the first time. The seasonal pattern has been maintained in 2014 with no Pf cases for two (April, May) of the first six months (personal observation of two AAH staff). Variation in climate is unlikely to account for this. Although there are no weather records for East Kwaio, the weather has not varied significantly over these years, being generally hot (> 26 °C) with at least 200 mm of rain per month.
Pv progressively became the dominant species over this time since its incidence fell less than Pf. Since primaquine is rarely used at AAH for vivax malaria, some of these cases could be relapses from hypnozoites. As malaria elimination progresses globally, Pv is becoming the predominant malaria species.16 The number of severe cases of malaria, as indicated by admissions, showed a similar spectacular decline, falling 91%. This was a conservative value since for the commencing year, 2008, three months of admissions data were missing, making the true reduction higher. Since the national malaria admission rate was 750 per 100 000 in 2008 and 1000 in 2012, the AAH rate of 1646 per 100 000 in 2008 was more than double, and the 2012 rate of 373 per 100 000 was a third of the national rate.8 Solomon Islands malaria-related mortality in 2007 and 2012 was 7 and 3 per 100 000, respectively.2 The AAH catchment area, with its malaria mortality rate of 54 per 100 000 was almost eight times the national rate in 2008 and much lower (zero) by 2012.
These results demonstrate that control of malaria in the Atoifi catchment area has exceeded the national performance. The API for Malaita Province in 2011 was 33.0, placing it sixth among the provinces.6 Atoifi achieved a lower API by 2013.
Prior to July 2009, malaria was treated with Fansidar with or without chloroquine. The AAH OPD records showed quinine being prescribed to initiate therapy on admission in 2008 and 2009 but not subsequently.
Causation cannot be attributed from such a study. However, the fall in malaria coincided with two significant events at AAH: (1) introduction of ACT as the only treatment available at OPD in July 2009; and (2) introduction of a long-term research capacity-strengthening project in 2009 that is still ongoing.17 ACT was introduced nationally in 2009 with variable results and not such a large decline in malaria incidence as reported in our study.8,18 The approach taken for the research capacity-strengthening activities at Atoifi is very inclusive, involving community members as well as health professionals and researchers.19 It includes community-based research of diseases of importance to the people of the area.20,21 This has made the AAH more accessible to the surrounding communities.17 Social capital is important in malaria control in Solomon Islands.22 The improved vertical social capital between the AAH and the local communities, which commenced in 2009, may have also contributed to the marked decline in malaria incidence. Indoor spraying is not conducted in this area and ITN use has not been assessed.The initial API for the study area was much higher than that reported for Solomon Islands nationally and for Malaita Province. In 2009, the API for Solomon Islands was 77.0 while the API for Malaita Province in the same year was the highest for the Solomon Islands at 82.9.23 The initial incidence of malaria in the Atoifi area was double the national API but similar to rates in the capital, Honiara.24 The villages with the highest APIs (Gounasuu, Wyfolonga and Abitona) are clustered within two kilometres of each other in a mangrove swamp, and vector-biting rates are probably high. However, there have been no entomological studies in this area. These villages can now be targeted for special attention by the malaria control programme at Atoifi. The API at Atoifi, the community which includes the AAH, was as high in 2008 as the APIs of the surrounding less well developed villages. This may have been due to Anopheles in the AAH being infected by patients attending the hospital and transmitting malaria locally. As a result of this finding, in late 2013 the hospital installed mosquito screens on the windows of all hospital wards. These examples highlight the value of calculating a village API.
Malaria was diagnosed by thick film only. This is the standard practice used in routine medical diagnostic laboratories in most developing countries. The accuracy of identifying Plasmodium species by thick film is less than by thin film.25 Since the same microscopists performed the majority of examinations; were trained in malaria microscopy; used a standard staining technique; had good quality, well-maintained microscopes; and results were provided to clinicians promptly, the trend data will be reliable.25,26 Atoifi also meets the standards of the Solomon Islands laboratory quality assurance programme and both microscopists are certified. Calculations for API and ABER were based on hospital data (numerator) and the total population of the administrative districts of Gulalofou and Waneagu/Taelanasina (denominator). Some people from very remote parts of these districts only travel to AAH for life-threatening illness or may bypass AAH to travel to health clinics in other districts.27 In addition, since the southern part of Ward 18 includes populations adjacent to Singalagu Harbour, residents may attend the Singalagu clinic instead of AAH, underestimating malaria incidence. However, we believe these factors have not changed over the period of the study and the trend data is reliable.
Although 10 weeks of data for 2009 were missing from the laboratory data, the available data had a high standard of accuracy with a low percentage of data missing. The admissions register was missing three months of data for the baseline year (2008) and for the treatment records in OPD. Additional problems with administrative data were illustrated by the lack of details in malaria treatment records. AAH has now requested OPD to record specific details of treatment given.
This study illustrates the value of analysing routinely collected administrative data to provide trends in disease. Laboratory results, hospital admissions and malarial treatments provided the indicators relevant to incidence and severity of malaria.
The RAP states that successful implementation of malaria programme activities is expected to result in six achievements by 2015.1 It is interesting to compare progress at Atoifi against five of these, bearing in mind that the RAP uses 2007 as a baseline and 2015 as the endpoint (Table 8).

The decline in the incidence and severity of malaria in the AAH catchment area has been spectacular, particularly for Pf. This was supported by three sources of hospital administrative data (laboratory, admissions and treatment records). The marked decline appears to be associated with the use of ACT and improved vertical social capital between AAH and the surrounding communities. Calculating village-specific API has highlighted which villages need to be targeted by the AAH malaria control team. The study illustrates the value of using routinely collected hospital administrative data in monitoring disease trends in a tropical resource-poor setting.
None declared.
This study received financial support from TDR, the Special Programme for Research and Training in Tropical Diseases, cosponsored by United Nations Children’s Fund, United Nations Development Programme, the World Bank and WHO (grant 1–811001688).