a National Centre for Communicable Diseases of Mongolia.
b Animal Health Project of Swiss Development Agency in Mongolia.
c National University of Medicine, Mongolia.
d Swiss Tropical and Public Health Institute, Basel, Switzerland.
Correspondence to Selenge Tsend (e-mail: tsendselenge2000@yahoo.com).
To cite this article:
Tsend S et al. Seroprevalence survey of brucellosis among rural people in Mongolia. Western Pacific Surveillance and Response Journal, 2014, 5(4). doi:10.5365/wpsar.2014.5.1.002
Background: After the transition from socialism to a market economy in 1990, human brucellosis re-emerged in Mongolia. The aim of our study was to estimate a representative seroprevalence of Brucella spp. and to determine risk factors for brucellosis seropositivity among rural people.
Methods: A cross-sectional study with multistage random selection was conducted in eight provinces of Mongolia. Study participants were interviewed using a questionnaire to obtain their brucellosis history, current symptoms and likely risk factors. Blood samples were drawn to determine brucellosis seroprevalence.
Results: A total of 2856 randomly selected rural people aged four to 90 years were enrolled in the study. The seroprevalence of Brucella spp. was 11.1% (95% confidence interval [CI]: 10.0–12.1), ranging between 2.3% and 22.6% in the eight provinces; 39.2% (n = 609) of nomadic camps had at least one seropositive participant. Risk factors associated with brucellosis seropositivity were being older than 45 years (adjusted odds ratio [AOR] = 6.9, 95% CI = 5.1–8.7) and being a veterinarian (AOR = 2.8, 95% CI = 1.5–5.0).
Conclusion: Our study confirms that human brucellosis seroprevalence among rural people in Mongolia is high. Human brucellosis can be effectively controlled if high-coverage livestock mass vaccination is implemented with a coverage survey after the vaccinations to ensure completeness. This mass vaccination should be accompanied by public awareness and educational programmes.
Brucellosis is a zoonosis, and the infection is almost invariably transmitted by direct or indirect contact with infected animals or their products. It is an important human disease in many parts of the world, especially in the Mediterranean countries of Europe, North and East Africa, the Middle East, South and Central Asia and Central and South America.1
Brucellosis is caused by members of the Brucella genus. Transmisson of infection to humans occurs through breaks in the skin, following direct contact with tissues, blood, urine, vaginal discharges, aborted fetuses or placentas.2 The most frequent symptoms of brucellosis are fever, chills or shaking, malaise, generalized aches and pains all over the body, joint and low back pain, headaches, anorexia, easy tiredness and general weakness.3
Mongolia has the second highest incidence of human brucellosis worldwide; another seven republics of the former Soviet Union are included in the 25 countries with the highest incidence. According to data from the National Statistical Office of Mongolia, a rapid increase in notified cases of brucellosis was observed between 1990 and 2000. The increase may have been the result of the evolution from a socialist state to a free market economy which led to the loss of rigorous livestock control.4 During this period, changes to the health system precluded early recognition of the disease or interventions that considered the emerging trends in humans and animals.5 In Mongolia, factors contributing to the incidence of brucellosis include traditional eating habits, standard hygiene measures, methods for processing milk and its products and rapid movement of animals.3
In 2011, a national brucellosis serosurvey was conducted that sampled 168 027 head of livestock from 11 528 nomadic camps (two to more than four herder families that share the same pasture and water source) of 337 districts of 21 provinces.6 Twenty-one provinces, 57.3% of all districts and 8.0% of all nomadic camps had seropositive livestock including camels, cattle, sheep and goats. Livestock seroprevalence was found in 0.7% of camels, 1.8% of cattle, 0.7% of sheep and 0.5% of goats using parallel interpretations of Rose Bengal Tests (RBT), complement fixation tests and competitive-enzyme-linked immunoabsorbent assay (ELISA).6
The aim of our study was to estimate the seroprevalence of Brucella spp. and to determine risk factors for brucellosis seropositivity among rural people.
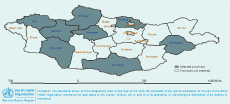
Eight provinces were selected for the cross-sectional surveys. Between June and September 2010, surveys were conducted in Sukhbaatar and Zavkhan provinces, selected for convenience.7 Between November 2011 and January 2012, the same surveys were conducted in a further six provinces: Arkhangai, Khuvsgul, Selenge, Uvs, Umnugovi and Govi-Altai (Figure 1). In each province, four districts were selected using simple randomization in Excel (the rand () command). Twenty nomadic camps and four to five individual participants were randomly selected based on the required sample size.

The cluster sample size calculation as described elsewhere7 assumed a human brucellosis seroprevalence among Mongolian rural people of 20%.8 In addition, the number of clusters and number of individuals per cluster was optimized according to the feasibility and the available budget.
The study was approved by the Ethics Committee of the Health Sciences University of Mongolia and the Ethics Committee of the Canton of Basel of Switzerland. All participants were informed about the study and what they could expect regarding diagnosis, reporting and treatment; all signed a consent form. A child younger than 16 years of age was included in the study with signed consent from of his/her parents.
All study participants were interviewed using a questionnaire which included demographics, risk factors and clinical symptoms for brucellosis. The questionnaire was pre-tested during the 2010 study in Sukhbaatar and Zavkhan7 and revised for the extended study to improve understanding of questions and to eliminate overly-sensitive questions.
Venous blood was taken with 5 ml Vacutainer® tubes. The blood samples were centrifuged in 3000 rounds per minute for five minutes. Separated 1.5 ml tubes of serum were kept in a cool box and transported to the provincial laboratories for storage and cooling before shipment to the serological laboratory of the National Center for Communicable Diseases in Ulaanbaatar where they were tested for brucellosis.
Sera were tested with the RBT for detection of antibodies to Brucella abortus/melitensis from Tulip Diagnostic Ltd (Bambolim, India). Positive sera were re-tested with the RBT using ½ to 1/32 serum dilutions,9 and with enzyme immunoassay for the qualitative determination of IgG class antibodies against Brucella from the NovaTec Immundiagnostica GmbH (Dietzenbach – 63128 Germany). The ELISA test was performed according to manufacturer’s instruction.
All data were double-entered in Access 2007, compared in Epi Info™ 3.5 to correct entry errors and analysed using STATA 10.1. Study participants who tested positive by either ELISA or RBT were considered seropositive for the statistical analysis.
To assess the association between risk factors and human brucellosis seropositivity we used Pearson χ2 or Fisher’s exact tests for explanatory variables such as demographics, behaviour-related risk factors and reported clinical symptoms. We also conducted univariate logistic regression using the binary serological outcome with the xtgee command and random effect on the nomadic camp level. A multivariate logistic regression model (with random effect at the nomadic camp) using backward stepwise selection and a removal level for covariates at P = 0.10 based on the likelihood-ratio test was then constructed. Variables with p values less than 0.05 in the univariate analysis were included in the multivariate model.
To determine the proportion of the general population seroconverting each year due to brucellosis exposure, the seroprevalence data were divided by the duration of seropositivity, assumed to be 10.9 years.10 Using a conservative estimate of 20% of seroconversions representing true clinical cases (note that among all seropositives detected, 58.5% had at least two symptoms and 31.5% had at least three symptoms at time of interview), these proportions were multiplied by 0.3 and converted to rates per 100 000 for the general population.
There were 2856 study participants from 609 nomadic camps from 31 districts in the eight selected provinces between four and 90 years of age (median 38 years). This included 2260 (79.1%) herders, 142 (5.0%) students, 96 (3.4%) office workers, 70 (2.5%) workers, 37 (1.3%) retired people, 20 (0.7%) veterinarians, 18 (0.6%) entrepreneurs, 16 (0.6%) unemployed adults, 13 (0.5%) children under six years, and 184 (6.4%) other residents.
The seroprevalence of Brucella spp. among participants was 11.1% (95% CI: 10.0–12.1) ranging from 2.3% to 22.6% in the eight provinces (Table 1) and 4.1% to 43.8% in the 28 districts. Within nomadic camps, 39.2% (95% CI: 38.2–41.0) had at least one to four seropositive members (Table 2). This equated to an annual incidence of seroconversion of 1145 per 100 000 and an overall annual incidence of 229 clinical cases per 100 000.
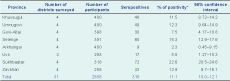
* Based on parallel interpretation of the RBT and ELISA test.
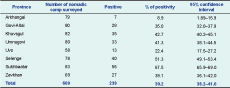
* Based on parallel interpretation of the RBT and ELISA test.
Seroprevalence was higher in females than in males (11.2% compared with 10.9%, P = 0.029). By age group, the highest seroprevalence was found in those 45 years and above at 15.5% (95% CI: 13.9–17.0), with the lowest in the four to 10 year age group at 2.6% (95% CI: 1.5–20.4). All occupation categories included seropositive cases ranging between 2.8% and 30.0% (Table 3).
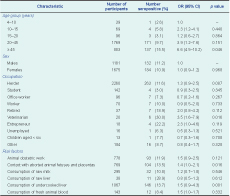
OR, odds ratio; CI, confidence interval.
* Based on parallel interpretation of RBT and ELISA.
Risk factors associated with being seropositive in univariate analysis included: being 45 years old and above (odds ratio [OR] = 6.6, P = 0.046), being a veterinarian (OR = 3.5, P = 0.016), contact with aborted animal fetuses and placentas (OR = 1.35, P = 0.016) and consumption of undercooked liver (OR = 1.51, P = 0.001) (Table 3).
In the multivariate analysis, only two variables remained associated with being seropositive: being 45 years old and above (adjusted odds ratio [AOR] = 6.9, 95% CI: 5.1–8.7) and being a veterinarian (AOR = 2.8, 95% CI: 1.5–5.0). Among veterinarians who participated in the study, 72.7% assisted in livestock obstetric work, and 50% had direct contact with aborted animal fetuses and placentas. The risk factors for veterinarians was also much higher compared with other occupations (P < 0.001).
Of the study participants, 2.7% (n = 76) reported receiving treatment for human brucellosis in the past; the median time since past brucellosis treatment was 14 years (Q1 = 3.3 and Q3 = 20 years). With the exception of testicular pain, there were significant differences between age groups in reporting clinical symptoms; the age groups of 20 to 44 years and 45 years and above reported more clinical symptoms for human brucellosis. Females also reported more headaches; joint, back and muscle pain; weakness and sleeping disturbances than males (Table 4).
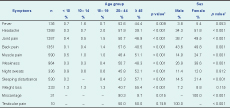
* Either derived from the χ2 test or Fisher’s exact test.
Reported clinical symptoms at the time of the study were compared to the sero-status of participants. Overall, 165 of the 316 (52.2%) brucellosis seropositive participants and 1186 of the 2540 (46.7%) seronegative participants reported symptoms. Among all seropositives, 36.7% reported more than three symptoms; among the seronegatives, 23.1% reported more than three symptoms (P < 0.001). Headache; joint, back and muscle pain; night sweats and sleeping disturbances were significantly associated with brucellosis seropositivity (Table 5).
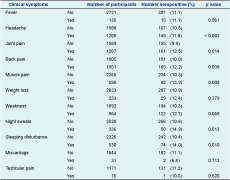
We report a seroprevalence of Brucella spp. among rural people of 11.1% (with a range between provinces from 2.3% to 22.6%) and an annual incidence of 229 per 100 000. The high incidence in the study likely reflects an increase in human brucellosis after the transition in Mongolia from socialism to a market economy leading to livestock privatization and collapse of the veterinary sector.4
Although several earlier studies also estimated the seroprevalences of Brucella spp. in Mongolia among high-risk people including herders, veterinarians and raw animal processing technicians,11–14 these differed from our study in time, study design and methodology and should not be compared. The result from our study was higher than the 0.1% to 10.1% reported among high-risk people in other countries,10,15–21 which is not surprising as Mongolia is ranked second in the world for brucellosis incidence.5 We also estimated a much higher incidence compared with that reported from notification data,22 despite the fact that we have taken a conservative assumption that 20% of seropositive cases are clinical cases.
According to the multivariate analysis, adults aged 45 years and above and veterinarians had a higher risk for brucellosis. This age group plays an important role in livestock herding and birthing, and veterinarians have direct contact with animals and aborted materials when doing veterinary examinations. We also found seropositives in all age groups, including in young children (four to nine years), which may indicate ongoing exposure and transmission of brucellosis in rural Mongolia. These groups should be targeted with material about protection against brucellosis infection.
This study will serve as a baseline of the seroprevalence of Brucella spp. in rural people in Mongolia before the implementation of a nationwide livestock vaccination campaign; it also will be used for ongoing brucellosis surveillance. A decrease of human incidence and repeated sero-surveillance surveys in humans will indirectly assess the efficacy of the vaccination campaign in livestock.23
There were several limitations to the study. First, association between human and livestock seropositivity was not assessed in provinces (with the exception of Zavkhan and Sukhbaatar7). There also may have been temporal variations in risk factors for childhood brucellosis, interpretation of reported clinical symptoms for brucellosis based on seropositivity and pathogen exposure that were not captured by the cross-sectional study design.
Our study confirms that human brucellosis seroprevalence among rural people in Mongolia is high and that the incidence is much higher than the notification data suggests. As recommended by the Food and Agriculture Organization of the United Nations, the World Organization for Animal Health and the World Health Organization, mass livestock vaccination is required in Mongolia in the mobile livestock production system.
Safety measures to avoid brucellosis include wearing protective clothes such as gloves, using metal hooks to collect aborted fetuses and placentas for burial or burning, washing hands after handling livestock and completely cooking liver from small ruminants. This information should be included in educational materials to prevent as many as possible new cases, especially at the beginning of the mass vaccination campaign while strains still circulate. We have developed written and pictorial educational materials mainly for children. The literacy rate in Mongolia is extremely high and thus printed media are appropriate. In parallel, the surveillance, treatment and diagnostic capacities for human brucellosis must be increased in provinces and districts. Education and awareness programmes should be implemented particularly before the livestock birthing season.
None declared.
The study was carried out in Sukhbaatar and Zavkhan provinces in 2010 with funding from the Swiss Agency for Development and Cooperation in Mongolia. We thank the Mongolian Ministry of Health, the Health Promotion Foundation of Mongolia and the Research Institute of Veterinary Medicine for funding the study in 2011–2012. We also wish to thank the staff of these agencies for their assistance on the study.
We would like to thank the health departments of the Arkhangai, Khuvsgul, Selenge, Uvs, Umnugovi, Govi-Altai, Zavkhan and Sukhbaatar provinces and districts, the physicians and the laboratory personnel for assisting with data collection.