
a Surveillance and Epidemiology Branch, Centre for Health Protection, Department of Health, Hong Kong (China).
b Field Epidemiology Training Programme, Hong Kong (China).
Correspondence to Wong Miu-ling (e-mail: mo_fetp2@dh.gov.hk).
To cite this article:
Wong M et al. An outbreak of community-associated methicillin-resistant Staphylococcus aureus infection in a boarding school in Hong Kong (China). Western Pacific Surveillance and Response Journal, 2014, 5(1):1–6. doi:10.5365/wpsar.2013.4.4.005
Background: In November 2012, an outbreak of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) skin and soft tissue infections affecting students at a boarding school in Hong Kong (China) was detected.
Methods: A case was defined as any student or staff notified with MRSA infection from 25 October 2012 to 5 July 2013 with the clinical isolate being of staphylococcal cassette chromosome mec type IV or V and positive for Panton-Valentine leukocidin gene. We conducted field investigations, advised on control measures and enhanced surveillance for skin and soft tissue infections at the school. Decolonization therapies were offered to all cases and contacts, and carrier screening was conducted.
Results: There were five cases; two (40%) were hospitalized and three (60%) required surgical treatments. Initial screening comprised 240 students and 81 staff members. Overall, four cases (80%) plus eight other students (3.3%) were carriers, with eight of 12 (66.7%) from the same dormitory. All staff members screened negative. After intensified control measures, the number of students screened positive for CA-MRSA decreased from nine to one with no more cases identified in the school.
Conclusion: Identification of carriers, decolonization therapy, monitoring of cases and contacts and strengthening of environmental and personal hygiene were control measures that helped contain this CA-MRSA outbreak in a boarding school in Hong Kong (China).
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) outbreaks in schools usually affect members of sports teams who come into bodily contact with one another. Considered as more virulent and transmissible than traditional MRSA strains,1 CA-MRSA may lead to outbreaks associated with severe morbidities and hospitalizations in otherwise healthy young adults or teenagers.2
CA-MRSA has been a statutory notifiable disease in Hong Kong (China) since 2007. Medical practitioners are required to report any patient with confirmed MRSA infection fulfilling the surveillance definition and to submit the culture isolate to a government public health laboratory for CA-MRSA confirmatory testing. The disease is rapidly emerging as annual numbers surged from 173 in 2007 to 813 in 2012. Most cases are sporadic skin and soft-tissue infections (SSTIs) with occasional clusters occurring in domestic settings.3
School X is a boys’ boarding school in Hong Kong (China). In addition to academic teachings, the campus has a marine activities centre, and students spend a significant amount of school time in water sports or training. There are about 250 students living in six dormitories (about 40 students in each one) with plenty of mixing activities among students during training and daily activities.
In October and November 2012, the Centre for Health Protection received three reports of CA-MRSA SSTIs among students from School X, which had no previous reports of CA-MRSA SSTI. Therefore, the case-based investigations were expanded to an outbreak investigation to determine the extent of the outbreak and to identify possible source(s) of infection. In this report, we present the outbreak investigation, including the implementation and outcome of control strategies.
A case was defined as any student or staff member of School X who was notified with SSTIs (e.g. boil, abscess and pustule) or other infections (e.g. pneumonia, sepsis) from 25 October 2012 to 5 July 2013, with MRSA isolated from any clinical specimen with the isolate being of staphylococcal cassette chromosome mec (SCCmec) type IV or V and positive for Panton-Valentine leukocidin (PVL) gene.
A carrier was any student or staff member of School X, without a clinical infection, who had MRSA isolated from any screening specimen collected from 25 October 2012 to 5 July 2013 with the isolate being of SCCmec type IV or V and positive for PVL gene. Cases were considered carriers if they had a positive screening result after their initial diagnosis.
Screening of students (after obtaining consent from parents/guardians) and staff from School X was conducted from 5 November 2012 to 22 March 2013. Initially limited to close contacts of the first notified case (e.g. students in same dormitory, contact-sport team members), screening was extended to all students and staff when the second case was notified (i.e. outbreak established) in November. Attack rates (AR%) by dormitory were calculated by dividing the number of cases and carriers identified by the total number of students in the dormitory, assuming the total number remaining constant during the investigation period.
Decolonization therapies were offered to cases and screened contacts regardless of carrier status. The five-day regimen comprised daily application of a 4% chlorhexidine gluconate solution as liquid soap and shampoo together with thrice daily application of a topical 2% mupirocin cream to nostrils bilaterally.
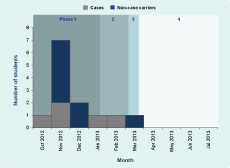
Results of decolonization therapies were assessed by post-decolonization screening: Phase 1 aimed to screen all students and staff once, and this occurred over four occasions from 5 November 2012 to 28 January 2013; Phase 2 occurred between 29 January and 12 March 2013, when post-decolonization screening of carriers and cases was completed; Phase 3 occurred from 13 to 22 March 2013, when all cases and Dorm A students were targeted; and Phase 4 occurred between 23 March and 5 July 2013, when the carriers identified in Phase 3 were re-screened (Table 1).

* Number screened positive in each phase included (a) those who remained in carrier status despite decolonization therapy offered in previous phase and (b) both cases and non-case carriers.
Nasal, axillary and perineal swabs were collected during the screenings and were sent to the Public Health Laboratory Service Branch for culture, PVL gene polymerase chain reaction (PCR), SCCmec typing, molecular spa-typing as well as antibiotic susceptibility tests.
Field investigations were conducted by the investigation team, infection-control nurses and a microbiologist. Mixing opportunities in school premises, hygiene facilities and practices were reviewed in each field visit.
From 25 March 2013, School X was requested to submit weekly reports of any skin lesions identified among students and staff to allow for early detection of potential new cases, timely referral for diagnosis, laboratory investigation and treatment.
Five cases were identified, aged between 13 and 16 years (median 15 years). Four lived in Dorm A (4/41, AR = 9.8%) and one in Dorm B (1/45, AR = 2.2%); both dormitories were located on the same floor. The first case developed symptoms on 14 October 2012, while the onset of the last case was on 18 February 2013. Two cases were diagnosed after initiation of the screening programme. Four cases presented with skin abscesses and one presented with a left arm pustule only. Two required hospital admission and three required surgical treatments such as incision and drainage. Their family members were all asymptomatic.
There were 254 students and 81 staff members at the school during the investigation period. Of these, 240 students (94.5%) (including the five cases) and 81 staff members (100%) were screened during Phase 1; two students refused screening and 12 were either absent or had quit the school. Two students refused decolonization therapies.
Overall, four of the five cases (80%) and eight other students (3.3%) were confirmed as carriers. Eight of these 12 carriers lived in Dorm A (8/41, AR = 19.5%), two in Dorm B (2/45, AR = 4.4%) and two in Dorm C (2/41, AR = 4.9%). Screening specimens from staff members were all negative.
During Phase 1, two cases and seven carriers screened positive. Two initial cases that screened negative in Phase 1 and two student carriers from Dorm A confirmed during Phase 1 re-screened positive in Phase 2, suggesting poor compliance to therapy and possibly ongoing disease transmission among Dorm A students. During Phase 3, when all Dorm A students and the five cases were re-screened, one new carrier was identified; two cases (one that tested positive and one negative in Phase 2) and one other carrier (re-screened positive in Phase 2) also were identified as carrying CA-MRSA. These four carriers were re-screened in Phase 4 with one again confirmed as a carrier.
In summary, the number of carriers for CA-MRSA decreased from nine to one (Table 1) over the screening phases; from 25 March 2013, no further CA-MRSA infection cases were identified (Figure 1).
CA-MRSA – Community-associated methicillin-resistant Staphylococcus aureus
All case isolates (n = 5) and all screening isolates (n = 12) were of spa type t441 and were resistant to erythromycin and clindamycin but were sensitive to gentamicin, vancomycin and mupirocin.
Seven field visits to the school occurred. Health talks during each field visit provided information to students and staff on disease, personal and environmental hygiene, advice on wound treatment and exclusion from sports. The school was advised to conduct terminal cleansing during the Easter holiday (28 to 29 March 2013) when all dormitories were vacated.
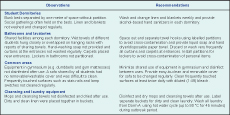
Substantial mixing opportunities in the dormitories, bathrooms, laundry and common areas (e.g. gymnasium) were identified. Deficiencies in hand-hygiene facilities and awareness and suboptimal environmental and personal hygiene were possible factors for CA-MRSA transmission in School X (Box 1). Staff of School X were also asked to supervise non-compliant students for decolonization therapy to intensify outbreak control.
We reported a CA-MRSA outbreak affecting five students in a boarding school in which two (40%) were hospitalized and three (60%) required surgical treatment; this was the largest institutional CA-MRSA outbreak recorded in Hong Kong (China). In the early phases of outbreak control, despite repeated field inspections, universal screening and decolonization therapies in the school, compliance to decolonization therapy and progress on environmental interventions remained suboptimal. Two cases initially screened negative in the first phase were detected as carriers in the second phase, indicating possible ongoing transmission.
A regimen of intranasal mupirocin and chlorhexidine body wash have been found to eradicate CA-MRSA colonization in more than 80% of carriers in Hong Kong (China).4 This decolonization regime was adopted early in the control of this outbreak, but compliance appeared to be poor as one new case and subsequent carriers were identified. Supervised decolonization therapy was then adopted as part of intensified measures together with reinforcement of environmental and personal hygiene control. Intensive cleaning of the school during the school holidays in March 2013 and weekly surveillance to ensure early identification and prompt treatment of potential skin lesions, as per a previously reported outbreak,2 were also adopted. The outbreak was contained after such coordinated efforts and interventions.
Previous local studies suggested that sharing of personal items is a risk factor, while good hand hygiene may protect against infection.5 In this outbreak, most cases (4/5, 80%) and carriers (5/8, 62.5%) lived in the same dormitory with shared use of facilities. Field investigations also revealed suboptimal hygiene practices which may have facilitated transmission within, and to a lesser extent between, dormitories in the school.
CA-MRSA isolates in Hong Kong (China) have been predominantly of spa type t019 and t437,6 different to the spa type t441 identified in this outbreak. However, the latter has occasionally been found in other Asian countries and is closely related to t437,7 belonging to the same lineage (sequence type 59, the Taiwan [China] clone).8
For future outbreaks, we recommend that systematic data be collected in each phase (e.g. hand hygiene and decolonization compliance) for quantitative analysis of the effectiveness of individual control measures.
We reported a CA-MRSA outbreak affecting five students in a boarding school in Hong Kong (China). Identification of carriers, decolonization therapy, intensive monitoring of cases and contacts and strengthening of environmental and personal hygiene were important strategies to help contain this school outbreak.
None declared.
None.
We would like to thank all staff of the Surveillance and Epidemiology Branch of the Centre for Health Protection who contributed in the investigation and control of this outbreak. We would also like to thank Alain Moren and Marta Valenciano for their advice in report writing.