a National Center for HIV/AIDS, Dermatology and STD, Ministry of Health, Cambodia.
b National Maternal and Child Health Center, Ministry of Health, Cambodia.
c Clinton Health Access Initiative, Cambodia.
d World Health Organization, Cambodia.
e Department of Public Health, School of Public Health and Health Sciences, University of Massachusetts Amherst, United States of America.
f School of Community and Global Health, Claremont Graduate University, United States of America.
g United Nations Children's Fund, Cambodia.
Correspondence to Masami Fujita (e-mail: fujitam@wpro.who.int).
To cite this article:
Sovannarith S et al. Uptake of interventions for preventing mother-to-child HIV transmission in 11 operational districts in Cambodia. Western Pacific Surveillance and Response Journal, 2012, 3(3):22-28. doi:10.5365/wpsar.2012.3.2.009
Introduction: To achieve the global goal of eliminating mother-to-child transmission of HIV, retention of HIV-positive women and their babies throughout the cascade of prevention of mother-to-child transmission of HIV (PMTCT) services is necessary. Little evidence has been published on coverage of the cascade in resource-limited settings. Along with PMTCT service expansion in Cambodia, a national routine reporting system was developed. This study examines coverage of six PMTCT interventions to improve our understanding of retention throughout the cascade.
Method: We developed indicators to monitor coverage of the six key interventions: (1) maternal antiretroviral treatment or prophylaxis; (2) delivery in a health facility; (3) infant ARV prophylaxis at birth; (4) infant co-trimoxazole prophylaxis at six weeks; (5) first infant DNA-PCR test at six weeks; and (6) second infant DNA-PCR test at 30 weeks. Programme data from April 2008 to December 2011 in 11 operational districts were used to identify those eligible for each intervention.
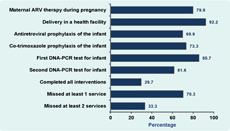
Results: Women eligible for maternal antiretroviral treatment or prophylaxis in the study were aged 18 to 48 with a median age of 30 years. Coverage of the six interventions were: (1) 79.9% (258/323); (2) 92.2% (236/256); (3) 69.9% (179/256); (4) 73.3% (184/251); (5) 85.7% (215/251); and (6) 61.6% (135/219). Among those eligible, 29.7% (65/219) received all six interventions.
Discussion: This study revealed critical gaps in PMTCT service delivery under routine conditions in Cambodia. Service optimization by reducing gaps will help eliminate HIV infection among infants and improve maternal survival. Further operational studies are needed to identify determinants of service uptake.
In 2010, an estimated 390 000 children globally were newly infected with HIV1 with an estimated 22 000 children from the Asia Pacific region newly infected with HIV in 2009.2 Over 90% of them were infected through mother-to-child transmission (MTCT). Without treatment, about half of children die before their second birthday.3 Without intervention, the risk of MTCT ranges from 20% to 45%. With specific interventions, the risk of MTCT can be reduced to less than 2% in non-breastfeeding populations and to 5% or less in breastfeeding populations. Despite the availability of effective interventions for prevention of mother-to-child transmission (PMTCT), much progress remains to achieve the global goal of virtually eliminating mother-to-child HIV transmission by 2015.4
In low- and middle-income countries, 35% of pregnant women received HIV testing and counselling in 2010, and only 48% of the estimated number of HIV-positive pregnant women received the most effective antiretroviral (ARV) regimens (excluding single-dose nevirapine) for PMTCT in 2010.1 In sub-Saharan Africa, the region with the highest number of pregnant women living with HIV, the coverage of HIV testing and counselling increased in 2010 but only reached 42% (up from 35% in 2009).1 In Asia and the Pacific, the coverage of HIV testing and counselling among pregnant women was even lower at 17% in 2009.5
In addition to HIV testing, counselling and ARV treatment or prophylaxis for pregnant women, the mother-infant pairs should be able to access a range of services throughout the PMTCT cascade including skilled care at birth, ARV and co-trimoxazole prophylaxis for infants and first and second DNA-PCR testing for infants. Opportunities to improve programme outcomes can be missed at each step of the PMTCT cascade. For example, in a study in Malawi, 55% of HIV-positive pregnant women were lost to follow-up by the 36-week antenatal visit, 68% by delivery and 81% by the six-month postnatal care visit.6 Similarly, according to a review paper, many newborn children are likely to be lost from care at each step of the early infant diagnosis process, "including infant presentation to care, test offer by healthcare professionals and test acceptance by parents/caregivers, specimen processing, result return to healthcare facilities and parents/caregivers, and linkage to care."7 In view of this, it is important to examine regularly the coverage of each service in the PMTCT cascade using available routine programme monitoring systems to understand at which stages retention is insufficient (or where there are significant drop-offs). Few papers have been published on the gains in coverage of each service of the PMTCT cascade using available routine data, particularly in low- and middle-income countries in Asia.
In Cambodia, one of the resource-limited countries in Western Pacific Region, HIV was first detected in 1991 and the first AIDS patient was diagnosed in 1993. The epidemic peaked in 1998 with an estimated HIV prevalence of 2% among adults aged 15 to 49, but successful interventions have dramatically curbed the epidemic.8 It is estimated that prevalence had fallen to 0.6% by 2010. In acknowledgement of its efforts to halt and reverse the HIV epidemic, Cambodia received a Millennium Development Goal Award in 2010. Also, HIV treatment has been rapidly expanded and the ART coverage among overall HIV-infected people reached more than 90% by the end of 2008.9 Remaining challenges for Cambodia include addressing concentrated HIV epidemics among sex workers, people who inject drugs and men who have sex with men, and moving towards elimination of new paediatric infections.
For PMTCT, only 29% of pregnant women received HIV testing and counselling in Cambodia in 2008 and of the total identified HIV-positive women only 27% received ARV.9 At that time, most of the PMTCT services were available only at selected antenatal/maternity health facilities co-located with voluntary counselling and testing sites. Subsequently, 179 (20%) of 903 health facilities providing antenatal care services also offered HIV testing and counselling. To improve the coverage of each step of the PMTCT cascade, the Cambodian Ministry of Health decentralized the HIV counselling and testing element of the PMTCT services to the health centre levels by adopting the Linked Response approach in two demonstration areas in 2008.10 The Linked Response aimed to strengthen existing reproductive health services and increase access to comprehensive HIV prevention, education, testing, care and treatment, including PMTCT services by establishing linkages between sexual and reproductive health and HIV services.
Following the successful demonstration of the Linked Response in the initial implementation areas, the approach was rolled out on a national scale, resulting in 921 (92%) out of 997 health facilities providing both antenatal care services and HIV testing and counselling to pregnant women. The coverage of HIV testing among pregnant women increased to 78.1% and that of ARV to 63.5% by 2011.11 According to the HIV sentinel surveillance in 2010, the HIV prevalence among pregnant women at antenatal care was estimated to be 0.4%. Building on the progress made in PMTCT, the Royal Government of Cambodia expressed its commitment to achieving elimination of new paediatric infections.5 Increased understanding of the remaining gaps in PMTCT service coverage is crucial to obtain the universal coverage levels required to achieve paediatric infection elimination. In parallel with the roll-out of the Linked Response approach, the Ministry of Health has worked to develop a cohort monitoring system that captures the data on service delivery throughout the PMTCT cascade from pregnancy to infant HIV diagnosis. This cohort monitoring system is being successfully implemented in a subset of operational districts.
In this study, we aim to use this cohort data to describe the coverage of six key PMTCT interventions, namely: (1) maternal antiretroviral treatment and prophylaxis; (2) delivery in a health facility; (3) infant ARV prophylaxis at birth; (4) infant co-trimoxazole prophylaxis at six weeks; (5) first infant DNA-PCR test at six weeks; and (6) second infant DNA-PCR test at 30 weeks.
This paper is based on routine programme data from April 2008 and December 2011 from the 11 operational districts where a complete set of data was made available from a total of 77 operational districts in Cambodia. Data were collected to measure the following indicators of the six key PMTCT interventions: (1) percentage of HIV-infected pregnant women identified who received ARV for PMTCT; (2) percentage of HIV-exposed infants who were delivered at health facilities; (3) percentage of infants born to HIV-infected mothers who received ARV prophylaxis at birth; (4) percentage of infants born to HIV-infected mothers who received co-trimoxazole prophylaxis at six weeks; (5) percentage of infants born to HIV-infected mothers who received the first DNA-PCR test at six weeks; and (6) percentage of infants born to HIV-infected mothers who received the second DNA-PCR test at 30 weeks.
The eligibility of the study participants for each of the six interventions was based on the National Guidelines for Prevention of Mother-to-child Transmission of HIV; the National Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected Children in Cambodia and the Standard Operating Procedures to Initiate a Linked Response for Prevention, Care and Treatment of HIV/AIDS, Sexually Transmitted Infection and Reproductive Health Issues.
All HIV-infected pregnant women identified during the study period were eligible for maternal ARV treatment or prophylaxis (Indicator 1) if they did not choose to have an abortion and their gestational age was at least 14 weeks by the time that the study ended. They included women who were reported as lost to follow-up or death. All infants born to HIV-infected women in the cohort served as denominator for Indicator 2 and Indicator 3. Infants born to HIV-infected women who reached six weeks of age by the time the study ended were eligible to receive co-trimoxazole prophylaxis (Indicator 4) and the first DNA-PCR test (Indicator 5). They included infants who were reported as lost to follow-up or death. Infants were eligible to receive the second DNA-PCR test (Indicator 6) if they reached 30 weeks of age by the time that the study ended. They included infants who were reported as lost-to-follow-up or death but did not include those who tested positive at the first DNA-PCR test.
The analysis was limited to the specified 11 operational districts among the total of 77 operational districts due to higher completeness and quality of data from these 11 sites compared with many of the other operational districts where there were much greater challenges in obtaining timely and complete facility reports. These 11 operational districts have all benefited from supplemental technical and financial support from development partners for both programme implementation and data collection while some of the other operational districts have not received such support.
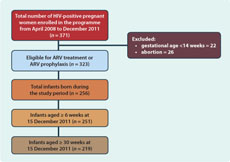
We extracted the routine programme data from the health facility registers. A total of 371 HIV-positive women including those diagnosed before the pregnancy or those newly diagnosed were enrolled into the programme. Of the women in the cohort, 323 were eligible for ARV treatment or ARV prophylaxis during pregnancy on the basis of their CD4 count and gestational age at diagnosis. Forty-eight women did not meet the criteria to receive ARV treatment or prophylaxis as they chose to have an abortion (n = 26) or their gestational age was less than 14 weeks (n = 22). A total of 256 children were born from 323 mothers who were eligible to receive ARV prophylaxis. Only 251 of these infants were eligible for co-trimoxazole prophylaxis and the first DNA-PCR test as they were at least six weeks old as of 15 December 2011. Of these 251 infants, 219 were eligible for the second DNA-PCR test at the age of 30 weeks (four children got HIV-positive result in their first DNA-PCR test at six weeks and 28 other children were not yet 30 weeks old) (Figure 1). A total of nine women died during the study period. All of these women received ARV treatment or prophylaxis and five gave birth to five babies. A total of 17 women were lost to follow-up throughout the cohort. Of these 17 women, 14 were lost to follow-up before treatment initiation.

For the analysis, we identified the number of HIV-positive women and children who received each of the above six interventions (numerator) and calculated coverage of these interventions using the total number of eligible HIV-infected women or children for each intervention as denominator (Figure 1).
All of the 323 eligible women were offered interventions to prevent HIV transmission from them to their children. Of them, 258 (79.9%) received ARVs (Figure 2). Women who were eligible for ARV treatment or prophylaxis included in the study were aged between 18 and 48 with a median age of 30 years old.
Note: The denominator for each indicator is the number of mothers or infants who were eligible for the intervention.
By 15 December 2011, 254 women in the cohort had given birth to 256 infants, as two women had twins. Of the 256 infants, 236 (92.2%) were born in health facilities and 179 (69.9%) received ARV prophylaxis after birth. Out of 251 eligible infants, 184 (73.3%) received co-trimoxazole prophylaxis and 215 (85.7%) received the first HIV DNA-PCR test. Similarly, 135 (61.6%) of the 219 infants who were eligible for the second HIV DNA-PCR test received it.
Of the 256 mother-infant pairs in the cohort, 251 were eligible for all five infant interventions when the infants were six weeks old. Of them, 93 (38.6%) completed each of the interventions. A total of 219 mother-infant pairs were eligible for all six interventions by the time the study closed. Of these, 65 (29.7%) completed all interventions, while 154 (70.3%) missed at least one intervention. Of the 256 newborn infants, 19 (7.4%) died; the cause and date of death of these children was not recorded.
Of the infants tested for HIV DNA, five were positive, including four positive at the first test (at six weeks of age) and one positive at the second test (at 30 weeks of age).
We identified critical service delivery gaps using the six coverage indicators of the PMTCT cascade in Cambodia. The Asia Pacific strategy for elimination of new paediatric infections defines the programme target for ARV coverage at 90% or above among the estimated total number of HIV-infected pregnant women and exposed infants by 2015.2 The denominator in this study was not all estimated HIV-infected pregnant women but all women who were accessing services; therefore, the target figures of the ARV coverage for this study population must be higher than 90% to move towards elimination. Similarly, coverage of other non-ARV services should be greater than 90% although no international or national targets have been set for them. This study revealed that none of the six interventions in the PMTCT cascade in the 11 selected operational districts in Cambodia have achieved that level of coverage yet. Furthermore, we found that the majority of the mother-infant pairs did not complete all the interventions. The suboptimal coverage of the full PMTCT cascade highlights the need for significant improvement in linked HIV and reproductive health service delivery.
In this study, about 80% of the identified HIV-positive pregnant women received ARV for PMTCT. This coverage is higher than the coverage in Zomba District, Malawi12 and in northern Uganda.13 In Malawi, a total of 75% of HIV-positive women who were not on ART received a single dose of nevirapine, while in Uganda, a total of 50% of HIV-positive women received either short-course zidovudine from the 36th week of pregnancy or single-dose nevirapine at the onset of labour or at least two hours before delivery. Although the uptake of ARV treatment or prophylaxis is higher among the HIV-positive women in our study than in others, further studies should explore the reasons behind this suboptimal access to ARV treatment or prophylaxis by HIV-positive pregnant women. The possible reasons may include lack of affordable transportation, mobility of the population, insufficient quality of counselling services in health facilities and fear of stigma and discrimination.
Over 92% of HIV-positive women who received ARV treatment or prophylaxis for PMTCT delivered at health facilities. This is an important achievement as this figure is much higher compared to the facility-based delivery rate (54%) in Cambodia among the pregnant women in the general population.14
Approximately 70% of the newborn children of HIV-positive women received the ARV prophylaxis. This result is much higher than the result of a study in Zimbabwe,15 in which only 31% of infants received ARV. However, the remaining 30% of the newborn children of this study who did not receive ARV prophylaxis were at high risk of acquiring HIV. It is documented that newborn children of HIV-positive women without ARV prophylaxis are more likely to get HIV infection from their mothers through breast feeding compared to those receiving ARV prophylaxis. Evidence shows that there is an incremental increase in the probability of mother-to-child HIV transmission of approximately 0.2% for each month of breastfeeding among those receiving triple ARV prophylaxis or treatment.16 The rate of transmission from mother to child with less effective regimens such as a single dose of nevirapine or no prophylaxis is as high as 1.57% among mothers with CD4 counts lower than 350.17
Among this group of HIV-exposed infants, about 73% received co-trimoxazole prophylaxis, reducing the risk of having opportunistic infections. Co-trimoxazole prophylaxis is safe and highly effective in reducing morbidity and mortality among HIV-positive children. The World Health Organization, therefore, recommended initiation of co-trimoxazole prophylaxis to all HIV-exposed infants at around six weeks of age.18 The infants who did not receive co-trimoxazole prophylaxis were at a higher risk of mortality in the absence of its preventive benefits.
Cambodian guidelines recommend HIV testing using DNA-PCR test (with dried blood spot) for all infants born to HIV-positive women at six weeks of age.19 However, about 14% of the children in our study did not receive the first test at six weeks of age. Similarly, about 38% of the newborn infants born to HIV-positive women did not receive the test at 30 weeks. This is a critical gap as such children may have missed the opportunity for early diagnosis in the event that they were infected by the virus during the postnatal period.
The most striking result of our study is that fewer than one-third of the HIV-positive women and their infants aged over 30 weeks completed all six key interventions of the PMTCT cascade. In a previous study in Malawi,12 only 18% of HIV-positive women completed all the recommended strategies i.e. both mothers and newborn infants took single-dose nevirapine and they followed the recommended feeding option. Although the proportion of mother and infant pairs who completed all the PMTCT services is higher in our study than other studies, our results clearly highlight critical service delivery gaps as well as the need for further studies in identifying the correlates of the uptake of each step of the PMTCT cascade.
One of the limitations of this study was the small number of operational districts included in the study because of the higher completeness and quality of routine programme data in these operational districts compared with the other operational districts in the country. It is possible that the selected operational districts have stronger programmatic capacity in implementing the PMTCT programme than other areas due to additional support received from development partners. The results of this study, therefore, may not be generalizable to the whole country. In Cambodia, the Ministry of Health has been working to improve the routine monitoring system of the PMTCT programme throughout the country. The cohort monitoring system is being implemented with relative success in these 11 operational districts. However, many districts will require further support to improve completeness and quality of data recording. Through these efforts it will be possible for the national programme to analyse this rich programme data reflecting PMTCT coverage throughout the service cascade. While our data comes from only a small sample of the total number of operational districts, the results from this analysis provide a preliminary indication of coverage in Cambodia and a potential baseline against which to measure Cambodia's progress towards scaling up access to ARVs for HIV-infected pregnant women and, eventually, elimination of new paediatric HIV infections.
In conclusion, this study revealed the critical gaps in PMTCT service delivery under routine programme conditions in Cambodia. Optimization of PMTCT services by reducing such gaps will help to eliminate HIV infection among newborn infants and improve maternal survival. Further operational research is needed to identify the determinants of the uptake of the PMTCT services.
None declared.
None.
The authors express their appreciation for the contribution of officials, health workers, nongovernmental organizations and civil society organizations in developing and expanding PMTCT services in Cambodia. Special thanks go to in-country experts of concerned partner agencies who contributed to the establishment of the PMTCT programme monitoring system, including the Clinton Health Access Initiative, the World Health Organization, the United Nations Children's Fund, the Joint United Nations Programme on HIV/AIDS, the United States Centers for Disease Control and Prevention, the United States Agency for International Development and FHI 360 in Cambodia.