|
Abstract
Adenosine-triphosphate-(ATP)-binding cassette (ABC) transport proteins are ubiquitously present membrane-bound efflux pumps that distribute endo- and xenobiotics across intra- and intercellular barriers. Discovered over 40 years ago, ABC transporters have been identified as key players in various human diseases, such as multidrug-resistant cancer and atherosclerosis, but also neurodegenerative diseases, such as Alzheimer’s disease (AD). Most prominent and well-studied are ABCB1, ABCC1, and ABCG2, not only due to their contribution to the multidrug resistance (MDR) phenotype in cancer, but also due to their contribution to AD. However, our understanding of other ABC transporters is limited, and most of the 49 human ABC transporters have been largely neglected as potential targets for novel small-molecule drugs. This is especially true for the ABCA subfamily, which contains several members known to play a role in AD initiation and progression. This review provides up-to-date information on the proposed functional background and pathological role of ABCA transporters in AD. We also provide an overview of small-molecules shown to interact with ABCA transporters as well as potential in silico, in vitro, and in vivo methodologies to gain novel templates for the development of innovative ABC transporter-targeting diagnostics and therapeutics.
INTRODUCTION
From MDR to neurodegeneration: ABC transporters in human disease
ABC transporters, Aβ proteins, and AD
PART I: STATUS QUO
ABCA transporters: Physiological function and implications for AD
ABCA1
ABCA2
ABCA3
ABCA4
ABCA5
ABCA6
ABCA7
ABCA8–ABCA10
ABCA12
ABCA13
Modulators of ABCA transporter function, trafficking, and regulation
Small-molecule interactors of ABCA transporters
Small-molecule regulators of ABCA transporters
PART II: PIPELINE DEVELOPMENT TO GAIN NOVEL DIAGNOSTICS AND THERAPEUTICS
In silico methodologies to predict novel lead structures
Structure-based drug design
Ligand-based drug design
In vitro methodologies to assess novel lead structures
Host system of ABCA transporters
Functional assessment of ABCA transporters
In vivo assessment of clinical candidates
Knock-out mouse models
RNAi models
Overexpression models
Humanized ABC transporter mouse models
Disease models
Imaging techniques
CONCLUDING REMARKS: WHERE DO WE GO FROM HERE?
APPENDIX
Abbreviations
5-FU - 5-fluorouracil, Aβ - amyloid-β, ABCA - ATP-binding cassette transporter subfamily A,
ACAT - acyl coenzyme A cholesteryl acyl transferase, AD - Alzheimer’s disease, ADMA - asymmetric dimethylarginine, ADP - adenosine-diphosphate, ALS - amyotrophic lateral sclerosis, AMPK - cAMP-activated protein kinase, APOA1/E3/E4 - apolipoprotein A1/E3/E4, APP - amyloid precursor protein, ATP - adenosine-triphosphate, BBB - blood-brain barrier, BCSFB - blood-cerebrospinal fluid barrier, BHK - baby hamster kidney, BIG1 - brefeldin 1-inhibited guanine nucleotide exchange protein, BODIPY - 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, cAMP - cyclic adenosine monophosphate, CFTR - cystic fibrosis transmembrane conductance regulator, CHO - Chinese hamster ovary, CNS - central nervous system, CPT-cAMP - 8-(4-chlorophenylthio)-cAMP, Cryo-EM - cryogenic electron microscopy, CSF - cer-ebral spinal fluid, DIDS - 4,4’-Diisothiocyano-2,2’-stilbenedisulfonic acid, EC50 - half-maximal effect concentration,
ECD - extracellular domain, ECGC - epigallocatechin gallate, ED50 - half-maximal effective dose, EOAD - early-onset AD, FPD5 - fluorescigenic pyrazoline derivative 5, FXR - farnesoid-X-receptor, GFP - green fluorescent protein, GGPP - geranylgeraniol pyrophosphate, GSH - reduced glutathione, GWAS - genome-wide association study, HD - Huntington’s disease, HDAC2 - histone deacetylase 2, HDL - high-density lipoprotein, HMG-CoA-reductase - 3-hydroxyl-3-methyl glutaryl-coenzyme A reductase, HTS - high-throughput screening, IC50 - half-maximal inhibition concentration, LAMP1 - lysosomal-associated membrane protein 1, LDLR - LDR receptor, lncRNA - long non-coding RNA,
LOAD - late-onset AD, LTC4 - leukotriene C4, LXR - liver-X-receptor, MDR - multidrug resistance,
mRNA - messenger RNA, MS - multiple sclerosis, MSD - membrane-spanning domain, NBD - 7-nitro-2,1,3-benzooxadiazole or nucleotide binding domain, NDEA - N-nitrosodiethylamine, NEM - N-ethylmaleimide, (ox)LDL - (oxidized) low density lipoprotein, PCB29-pQ - 2,3,5-trichloro-6-phenyl-[1,4]-benzoquinone, PD - Parkinson’s disease, PDB - protein data bank, PG-J2 - prostaglandin J2, PMA - phorbol 12-myristate 13-acetate, PPAR - peroxisome proliferator-activated receptor,
PRDX1 - peroxiredoxin 1, RAR - retinoic acid receptor, RNA - ribonucleic acid, RXR - retinoid-X-receptor, SAR - structure-activity relationships, shRNA - short-hairpin RNA, siRNA - small interfering RNA, SNP - single nucleotide polymorphism, SR-BI (Srb1) - scavenger receptor B1 (also HDL receptor), SREPB - sterol regulation element-binding protein, TKI - tyrosine kinase inhibitor, TKI - tyrosine kinase inhibitor, TM - transmembrane helix
INTRODUCTION
From MDR to neurodegeneration: ABC transporters in human disease
ABC transporters are membrane-bound transport proteins that are ubiquitously present in the human body.1-4 They play a major role in determining the distribution of intrinsic and xenobiotic drugs between intra- and intercellular compartments.5,6 The clinical relevance of ABC transporters became pronounced when their expression was correlated to cross-resistance of cancer cells to antineoplastic agents.3,7-13 This phenomenon is called ‘multidrug resistance’ (MDR). However, despite enormous efforts and countless clinical trials to target these efflux pumps,14-17 MDR is still a major unresolved obstacle in cancer chemotherapy. To date, most ABC transporters have been associated with MDR,3,7-9,11,12 but only a small minority has been studied properly and can be addressed by small-molecule modulators.18-22 Amongst these are ABCB1,1,18-27 ABCC1,1,18,19,23,24,26,27 and ABCG2.18,19,25
Apart from their role in multidrug-resistant cancer, many ABC transporters have been identified as key players in neurological disorders. Evidence for this includes their high abundance at the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) in the central nervous system (CNS).28-32 Additionally, their expression is altered in many pathological conditions in the brain.28-30,33-40 Important players are, again, ABCB1,28-30,34-36,39-44 ABCC1,28-30,39,41,43,45 and ABCG228,30,34,36,39-41,43in diseases like AD,28-30,41 amyotrophic lateral sclerosis (ALS),34,36,44 encephalopathy,45,46 epilepsy,39,40 multiple sclerosis (MS),35 and Parkinson’s disease (PD).42,47 Furthermore, ABC transporters were also found to be associated with certain genetic neurological and psychiatric diseases such as Huntington’s disease (HD),38 bipolar disorder,48,49 depression,48 or schizophrenia.48,49 Table 1 summarizes the involvement of ABC transporters in neurological diseases.
| ABC transporter |
Associated diseases |
| ABCA1 |
AD50
HD51
|
| ABCA2 | AD52
abnormal sphingolipid metabolism53,54
| | ABCA4 | cone-rod dystrophy55
fundus flavimaculatus56
retinitis pigmentosa57,58
Stargardt disease59-62
| | ABCA5 | AD28
| | ABCA7 | AD63
| | ABCA13 | Lewy body disease64
psychiatric disorders48,65,66
stroke in mice67
| | ABCB1 | AD28
brain tumors68
HIV-associated depression and schizophrenia69,70
HIV-associated encephalopathy46
epilepsy71
ischemic stroke72
MS35
multiple systems atrophy73
PD74
progressive supranuclear palsy75
Creutzfeldt-Jakob disease76
| | ABCB7 | PD77
| | ABCB9 | PD78
| | ABCC1 | AD28
brain tumors79
epilepsy39
HIV-associated encephalopathy45
ischemic stroke80
| | ABCC2 | brain tumors79
epilepsy39
| | ABCC3 | brain tumors79
epilepsy39
| | ABCC8 | ALS81
| | ABCC9 | ALS81
limbic-predominant age-related TDP-43 encephalopathy (LATE)82
hippocampal sclerosis of aging and depression83
| | ABCD1 | cerebral adrenoleukodystrophy84
| | ABCG1 | AD85
brain metabolic disorder86
| | ABCG2 | AD87
ALS88
brain tumors89
epilepsy90
MS91
PD47
traumatic brain injury92
| | ABCG4 | AD93
HD51 |
ABC transporters, Aβ proteins, and AD
Since 2001, ABC transporters have been implicated in AD pathogenesis.28-30,41,43,94,95 Specifically ABCB1,94 ABCC1,96 and ABCG297 have been suggested to directly transport amyloid-β (Aβ) proteins, being involved in Aβ clearance from the brain to the blood stream.94,96,97 In light of the failure of the first immunological treatment studies,98 it was already proposed that ABC transporter dysfunction could explain the clearance problem of Aβ.99,100 Cerebral accumulation of Aβ proteins interferes with neuronal metabolite homeostasis and leads to interruption of cortico-cortical circuits and hampered synaptic communication. This results in an irreversible atrophy and degeneration of specific brain regions, which further causes behavioral, cognitive, and visuospatial impairments in the progression of AD.101
The most prominent ABC transporter subfamily involved in AD is the ABCA subfamily of cholesterol and phospholipid transporters, in which particularly ABCA1, ABCA2, ABCA5, and ABCA7 have been associated with AD.28-30,41,43,95,102 For ABCA1,28,41,95,103 and specifically for ABCA7,28,41,95,104-107 genetic variant28,41,108-111 and genome-wide association studies (GWAS)28,41,106,107,112 have suggested that these transporters are risk factors in AD. These discoveries give the members of the ABCA subfamily a special standing within the group of AD-related ABC transporters.
Cholesterol metabolism in the context
of AD has been discussed extensively before.95,102,104,105,113-116 The contribution of cholesterol and phosphilipid transport to membrane constitution, composition, fluidity, and lipid raft formation mediated by ABCA transporters has already been proposed,6 presenting a putative pharmacological target.117 Targeting cholesterol and lipid distribution impacts Aβ production by differential activities between α-, β-, and γ-secretases, but also amyloid precursor protein (APP) processing106,118-122 and Aβ degradation.106,119,123-126 A contribution of ABCA transporters to Aβ clearance from the brain was also proposed,103,106,119,124,127 but not through direct Aβ transport.128,129
Although ABCA transporters have been reviewed for the last two decades,3,130,131 little is known about their specific contribution to AD pathogenesis and their mode of action. This is mainly due to a lack of small-molecules that can be used to track, study, and impact the function of these under-studied ABC transporters.
The present review consists of two parts:
PART I provides the status quo of ABCA transporters in AD and small-molecule modulators – in particular intrinsic substrates, natural compounds, pharmacological drugs, and synthetic molecules – that have been reported to influence ABCA transporter function and expression; PART II outlines the necessary drug development pipeline for the discovery of novel lead structures as potential innovative diagnostics and therapeutics against AD. This pipeline includes cutting-edge in silico methodologies, established in vitro cell assays, and necessary in vivo models.
Collectively, this review contributes to a deeper understanding of small-molecule ligands that influence ABCA transporter function, potentially leading to the development of novel AD diagnostics and therapeutics.
PART I: STATUS QUO
ABCA transporters: Physiological function and implications for AD
ABCA transporters are ubiquitously present in the human body,3,10,13 although differentially expressed.10 All of the 12 subfamily members have been associated with cholesterol and/or phospholipid transport and homeostasis,3,13,132 except for ABCA4, which is primarily a transporter of retinoids.133-138
In addition to the diseases listed in Table 1, ABCA transporters have been described as key proteins in several other human disorders, including neonatal respiratory distress syndrome (ABCA3),139 chronic interstitial lung disease (ABCA3),140 cataract-microcornea syndrome (ABCA3),141 hypertrichosis terminalis (ABCA5),142 or Harlequin ichtyosis (ABCA12).143
However, one major clinical implication for ABCA transporters, particularly ABCA1, ABCA2, ABCA5, and ABCA7, relates to AD.28,50,52,63 Their suggested roles in this major burdensome neurodegenerative disease as well as general physiological aspects are summarized in the following sections.
ABCA1
ABCA1 is the prototype of the ABCA subfamily,144 was first identified in 1994, and is located on human chromosome 9.145 The complete genomic sequence of human ABCA1 was reported in 2000. The ABCA1 gene spans 149 kb comprising 50 exons, and the resulting protein is 2261 amino acids long.146 ABCA1 is located in the plasma membrane and is also present intracellularly in the endoplasmic reticulum and Golgi apparatus, where it mediates the efflux of cholesterol and phospholipids from intracellular compartments to extracellular lipid-free apolipoproteins, mainly apolipoprotein A1 (APOA1) and to a lesser extend APOA2 and APOE, to form high-density lipoprotein (HDL) particles.3,147,148 The lipidation of APOA1 is preceded by ABCA1 dimerization.149 ABCA1 thus represents the first and rate-limiting step in the reverse cholesterol transport pathway, which removes excess cholesterol from peripheral tissues via HDL and delivers it to the liver for conversion into bile acids and subsequent excretion. In contrast to peripheral tissues, the physiological role of ABCA1 in the brain, where it is expressed in all cell types, is not well defined.103 It has been suggested that ABCA1 is required for cholesterol transport from glial cells to neurons via APOE, which is secreted by glial cells and serves as the main lipid acceptor in the brain.103,125 In vitro and in vivo studies in Abca1 knock-out models demonstrated that ABCA1 is essential for normal APOE secretion and lipidation in the CNS.150,151 Glial cells deficient for ABCA1 showed reduced lipid efflux with concurrent lipid accumulation as well as decreased APOE secretion, with APOE particles being small and poorly lipidated. In mice, Abca1 knock-out resulted in dramatically decreased brain levels of APOE. Moreover, examination of the hippocampi of Abca1-deficient mice revealed a decrease in neurite length and number of neurite segments and branches, pointing to an importance of ABCA1 for neurite integrity.152
The major genetic risk factor for sporadic AD is the allelic state of the APOE genotype, with inheritance of the APOE4 allele markedly increasing disease risk.153,154 Recently, Rawat et al. investigated how APOE4 affected ABCA1 expression and function in vitro in astrocytes.155 The authors found that APOE4 decreased ABCA1 plasma membrane levels and increased ABCA1 co-localization with late endosomes via activation of ADP-ribosylation factor 6, thereby reducing cholesterol efflux and lipidation of APOE particles. They corroborated their findings in blood-cerebrospinal fluid (CSF) showing that CSF from homozygous carriers of the APOE4 allele was less efficient in stimulating ABCA1-mediated cholesterol efflux compared to CSF from homozygous carriers of the APOE3 allele.
A recent study assessed cholesterol efflux capacity of CSF by analyzing AD patients, non-AD patients, and control subjects.156 The results demonstrated that ABCA1-mediated CSF-cholesterol efflux capacity was markedly reduced in AD but not in non-AD demented patients. However, this difference did not depend on APOE4 status. Interestingly, ABCA1-mediated CSF-cholesterol efflux capacity inversely correlated with total and phosphorylated protein tau, suggesting a link between the dysfunction of HDL-like particle in CSF and neurodegeneration.
Apart from the indirect link via APOE, a direct link between ABCA1 and AD has also been subject to investigation. Expression of hippocampal ABCA1 was elevated on both the mRNA and protein levels and was positively correlated with neuro-pathological changes and dementia severity in AD patients.157 The authors of this study suggested that the observed upregulation of ABCA1 could be interpreted as a compensatory attempt to clear Aβ from the brain. Moreover, a variety of studies investigated associations between single nucleotide polymorphisms (SNP) in the ABCA1 gene and the risk for AD,28,108-111 reporting inconclusive results.95,103 A meta-analysis of several studies identified the ABCA1 rs2422493 (C477T) polymorphism as a risk factor for AD while no association was found for the rs2066718 (V771M) or rs1800977 (C14T) polymorphisms.111 This risk effect for rs2422493 was confirmed in a recent genetic variant association study that, in contrast to the meta-analysis, also reported an increased AD risk for rs2066718 and a decreased AD risk for rs1800977.109 Further genetic association studies and meta-analyses are necessary to search for potential associations between ABCA1 polymorphisms and AD risk.
In a recent AD GWAS, the rs1800978 polymorphism in the ABCA1 gene was identified as the lead SNP in a new genome-wide significant locus.158 The association of genetic variants of the ABCA1 gene with AD risk was confirmed by exome sequencing data analysis from 32,558 individuals.158 The study identified around 120 variants that have an increased frequency in early-onset AD (EOAD; 1.5%) and late-onset AD (LOAD; 1.1%) cases, compared to 0.5% of all controls. The data demonstrated that AD-association was mainly explained by extremely rare variants, but also by a smaller number of more common variants, e.g., N1800H.159 Intriguingly, loss of function and missense variants in the ABCA1 gene were respectively associated with a 4.7-fold (95%CI 2.2-10.3) and 2.7-fold (95%CI 1.9-3.8) increased EOAD risk, and this was lower for LOAD cases suggesting that the burden of damaging ABCA1 variants was concentrated in younger AD patients.
Additionally, some long non-coding (lnc) RNAs such as lncRNA LOC286367 have been shown to affect ABCA1 expression.160 LncRNA LOC286367 and ABCA1 are located on the same chromosome but are transcribed in opposite directions. A recent study demonstrated that LOC286367 reduces ABCA1 expression in THP-1 macrophages and increases the levels of proinflammatory cytokines.160
The role of ABCA1 in Aβ deposition and clearance as well as in Aβ deposits-related memory deficits has been extensively investigated in APP-transgenic mouse models of AD. The lack of ABCA1 decreased brain APOE levels and either did not affect or increased Aβ load.161-163 A recent study utilizing shotgun lipidomics experiments demonstrated a common APOE isoform-specific phospholipid signature between human APOE3/3 and APOE4/4 AD brains and lipoproteins isolated from astrocyte-conditioned media of APOE3 and APOE4 mice.164 Interestingly, the lipoproteins derived from wild-type and Abca1het mice had phospholipid content APOE3 > APOE4 > APOE3het > APOE4het suggesting that the combination of ABCA1 insufficiency and APOE4 genotype decreases APOE lipidation even further, thus aggravating APOE4 effect. These findings suggest that poorly lipidated APOE may promote Aβ aggregation.129,161-163 In contrast, overexpression of ABCA1 in an APP-transgenic mouse model resulted in increased lipidation, albeit reduced brain levels of APOE and decreased Aβ load, implying that highly lipidated APOE may reduce Aβ aggregation propensity.127 This is supported by findings of Deane et al., who showed that different APOE isoforms may differentially disrupt Aβ clearance from mice brains.165 A stable isotope-labelling kinetic study in an APP-transgenic mouse model either lacking ABCA1 or overexpressing ABCA1 demonstrated increased APOE clearance in both Abca1 knock-out and ABCA1-overexpressing mice, but did not reveal any effect on Aβ clearance or production, suggesting that ABCA1 may regulate Aβ deposition by a mechanism other than altering Aβ metabolism.166 In contrast, a study assessing the clearance of intracerebrally injected 125I-Aβ from the brain reported that Abca1-deficiency decreased Aβ clearance in non-APP-transgenic mice.167 Furthermore, knock-out of Abca1 was found to augment the dissemination of intracerebrally injected, brain-derived Aβ seeds in APP-transgenic mice.167 Haplodeficiency of Abca1 led to decreased brain APOE levels and increased Aβ oligomer levels but did not affect Aβ deposition in APP-transgenic mice.168 However, both haplodeficiency and homozygous knock-out of Abca1 aggravated cognitive deficits in APP-transgenic mice.152,167,168 Lastly, the lack of one copy of Abca1 exacerbated memory deficits, decreased Aβ clearance, and increased Aβ load in APP-transgenic mice expressing human APOE4 but not in APP-transgenic mice expressing human APOE3.169
ABCA2
ABCA2 is predominantly, but not exclusively, expressed in the brain, where it can be found in glial cells and neurons.170-173 On the subcellular level, ABCA2 is located in endo- and lysosomal membranes, facilitating the sequestration of waste substances into intracellular vesicles.172 In addition, it is involved in myelin lipid transport, neural development, and macrophage activation.30,174,175
Genetic variations of ABCA2 were identified as a risk factor for EOAD and sporadic AD.52,176 These two studies showed a strong correlation between rs908832 and AD.52,176 However, a later study could not find a link between this SNP and any form of AD.177 In addition, ABCA2 mRNA expression was upregulated in AD patients compared to controls suggesting ABCA2 as a biomarker for differential diagnosis of AD.178 Preclinical studies of ABCA2 suggested that this transporter modulates Aβ production via the LDL receptor (LDLR).179,180 ABCA2 overexpression increased LDLR density, and LDLR deficiency has been described to enhance Aβ deposition.181 Chen et al. reported a co-localization of ABCA2 and Aβ as well as Aβ upregulation in cells overexpressing ABCA2. In addition, impairment of ABCA2 expression using small interfering RNA (siRNA) was accompanied by a decrease in Aβ production.182 Abca2 depletion has been shown to induce a shift from β- to α-secretases and thus, a reduction of APP processing by γ-secretase.182 Furthermore, ABCA2 has been proposed to play a role in Aβ production as it has been reported to upregulate sphingosine in murine cells and, therefore, to induce APP transcription.183 However, another study in human cells could not confirm the modulation of Aβ production or cholesterol efflux by ABCA2.184 Thus, further research on the role of ABCA2 in AD pathogenesis and its potential as a therapeutic target is necessary.
ABCA3
Despite its initial report of exclusive lung expression,185 ABCA3 is also found in other tissues including the brain.186,187 Within the brain, the highest levels of ABCA3 were found in oligodendrocytes.188
ABCA3 plays a role in producing surfactants in the lung, suggesting that the transporter
may also be involved in lipid metabolism in the brain, specifically phosphatidylcholine and phosphatidylglycerol transport. Interestingly, phosphatidylcholine has also been discussed in the context of AD.189 A genetic study revealed that mutations in ABCA3 can also cause cataract-microcornea syndrome, a rare congenital malformation of the eye.141 The actual implications of the potential connection between altered ABCA3 functionality and AD need to be addressed in future studies.
ABCA4
ABCA4 is mainly expressed in the retina with very little presence in other tissues of the CNS.190 ABCA4 mutation causes Stargardt disease, characterized by macular dystrophy, retinal alterations, and lipofuscin accumulation.60,61,190,191 Other retinal diseases, such as fundus flavimaculatus, retinitis pigmentosa, or cone-rod dystrophy, have also been associated with mutations of ABCA4.55,57,58,192 ABCA4 is expressed in brain capillary endothelial cells, as well.193 However, no link between ABCA4 and AD has been suggested to date.
ABCA5
ABCA5 is a little-known member of the ABCA subfamily expressed mainly in skeletal muscle with unknown function in the brain.194 Studies in peripheral tissues suggest that the function of ABCA5 is associated with cellular lipid metabolism.195 Abca5 knock-out in mice induced signs of lysosomal storage disease in the heart and the thyroid gland.131
In the brain, ABCA5 is expressed in neurons and, to a lesser extent, in microglia, astrocytes, and oligodendrocytes.195 Fu et al. showed that ABCA5 stimulated cholesterol efflux in neurons and induced a decrease in Aβ production probably affecting APP processing but not its expression.195
ABCA6
ABCA6 is ubiquitously expressed with high levels in liver, lung, heart, brain, and ovaries. This transporter is probably involved in macrophage lipid homeostasis as it is upregulated during macrophage differentiation and is responsive to cholesterol treatment.196 Although certain missense variants of ABCA6 have been correlated with blood cholesterol levels,197 no link between ABCA6 and AD has yet been found.
ABCA7
ABCA7 was first identified in the year 2000, and is located on human chromosome 19.198-200 Analysis of ABCA7 mRNA expression levels has shown that this transporter is mainly confined to the brain and the immune system.3 Due to its high homology to ABCA1 (54%),200 ABCA7 was first hypothesized to play an important role in lipid trafficking, mediating cholesterol and phospholipid efflux. ABCA7 actively transports phosphatidylcholine, phosphatidylserine, and sphingomyelin from the cytoplasm to the exocytoplasmic leaflet of membranes.198,199,201 However, in contrast to ABCA1, ABCA7 generates only small HDL particles.202 Recent research has shown that lipid trafficking by ABCA7 plays a secondary role. Studies in Abca7 knock-out models have demonstrated that ABCA7 is involved in the phagocytotic activity of macrophages and fibroblasts198,203-205 but not in cell cholesterol release.206-208
In 2011, Hollingworth et al. identified the ABCA7 gene as an AD risk locus.198,209 In multiple studies, variants of ABCA7 have been associated with an increased risk of developing AD.198,210-212 In 2015, Steinberg et al. reported that rare loss-of-function variants of ABCA7 confer a risk of AD in Icelanders (odds ratio: 2.12; P = 2.2 ∙ 10-13), and found a similar association in study groups from Europe and the United States (combined odds ratio: 2.03; P = 6.8 ∙ 10-15).213 In particular, the rare AD-related polymorphism rs200538373 was associated with an AD risk odds ratio of 1.9.210 These studies suggest that reduced levels of ABCA7 may increase the risk of AD. Nonetheless, it is not clear how these polymorphisms affect ABCA7 function and contribute to AD progression. Increased levels of ABCA7 expression were described in AD patients and were also positively correlated with cognitive decline.198,211 This finding is consistent with Abca7 mRNA transcription levels in J20 mice.123 The increase of ABCA7 may be a compensatory defense mechanism that is insufficient to stop disease progression. Furthermore, the rs3764650G allele has been associated with increased neuritic plaques in human patients198,214 and a limitation of the neuroprotective effects of exercise intervention.215 These studies support a potential protective role of ABCA7 in AD. To date, three potential roles have been identified for ABCA7 contribution to AD: APP processing, immune response, and lipid metabolism.
Chan et al. proposed an inhibitory effect of ABCA7 on Aβ deposition after showing in vitro inhibition of Aβ production independent of β-secretase activity.120 Other authors proposed that ABCA7 is not directly linked to Aβ production, but rather through lipid metabolism as ABCA7 mediates the transport of lipids across the BBB and ABCA7 loss of function may alter cholesterol transport by decreasing APOE secretion and ABCA1 expression. This alteration in cholesterol metabolism can also contribute to AD development.216 However, Abca7 knock-out induced an increase of Aβ load with no difference in clearance rate and an increase of β-secretase expression. On the other hand, ABCA7 overexpression led to diminished Aβ production and improved cognitive function.217,218
Nevertheless, ABCA7 is highly expressed in phagocytic cells, including macrophages and microglia, suggesting a role of the transporter in phagocytosis.188,198 Phagocytosis is crucial to maintain brain homeostasis. Indeed, ineffective phagocytosis may induce neuroinflammation, which is a risk factor in AD. In addition, microglial cells are involved in phagocytosis and degradation of Aβ. Thus, an involvement of ABCA7 in microglial phagocytosis of Aβ may explain the contribution of this transporter to AD pathogenesis. In AD patients, increased ABCA7 transcription has been found in areas with plaques but not in unaltered regions such as the cerebellum.123 This increase in transcription was paralleled by microglia recruitment supporting the contribution of ABCA7 to microglia-mediated phagocytosis of Aβ. In addition, Abca7 knock-out mice showed a reduced microglia response after intracerebral Aβ injection.123 Kim et al. demonstrated an increased Aβ load in J20/A7 knock-out mice compared to J20 mice, potentially due to an altered phagocytic function.124,198 Furthermore, it has recently been shown that Abca7 haplodeficiency disturbs the microglial immune response and causes enhanced Aβ accumulation in microglia, probably due to alterations in endolysosomal trafficking.219
Last, a new hypothesis has emerged recently, assigning ABCA7 a prominent role in the altered lipidostasis hypothesis in AD.104 The authors of this study proposed the existence of a neurodegenerative lipid that is naturally removed by ABCA7. A loss of ABCA7 function due to the described polymorphisms might accelerate accumulation of this lipid, inducing Aβ aggregation. In fact, a link between cholesterol metabolism and ABCA7-mediated phagocytosis has been reported, which may also explain the protective properties of statin treatment in the development of AD.105,198,203,220
Despite recent findings, the role of ABCA7 in AD pathogenesis remains unclear. According to in vitro and preclinical research, it may be associated with phagocytic activity by microglia, which could be linked to cell cholesterol metabolism.105,198,203 Thus, further investigation is required to reveal the role of ABCA7 in AD pathogenesis and its potential use as a therapeutic target for this neurodegenerative disease.
ABCA8–ABCA10
So far, no obvious role of ABCA8–10 has been elucidated for AD, neurodegenerative diseases, nor any human disease. However, several potential intrinsic substrates of ABCA8 have been identified.10,221,222 Furthermore, a significant number of ABCA transporter modulators have been identified on this target.222 Hence, ABCA8 represents a good model system for the development of potential therapeutics targeting other ABCA transporters taking the scarce knowledge on this transporter subclass into account.
ABCA12
ABCA12 is expressed predominantly in the epidermis, and its main function is the transport of lipids.223 It is hypothesized that ABCA12 plays a role in skin lipid homeostasis. Mutations in this gene are associated with lamellar ichthyosis type 2 and Harlequin ichthyosis.143,224,225 However, a Japanese study investigated common polymorphisms of ABCA12 and did not find an association with sporadic AD.226
ABCA13
ABCA13 is the largest ABC transporter with 576 kDa.227 It has been reported to be highly expressed in the brain as well as in peripheral tissues.227 A very small study found reduced neuroinflammation and altered ABCA13 expression in post mortem analyses of brains from patients with Lewy body dementia.64 In addition, increased ABCA13 expression has been reported after stroke in mice.67 Furthermore, two studies showed enhanced ABCA13 mRNA expression in schizophrenic patients after different antipsychotic treatments, suggesting a role of this transporter in psychiatric disorders.48,65,66 However, no association between ABCA13 and AD has been found.
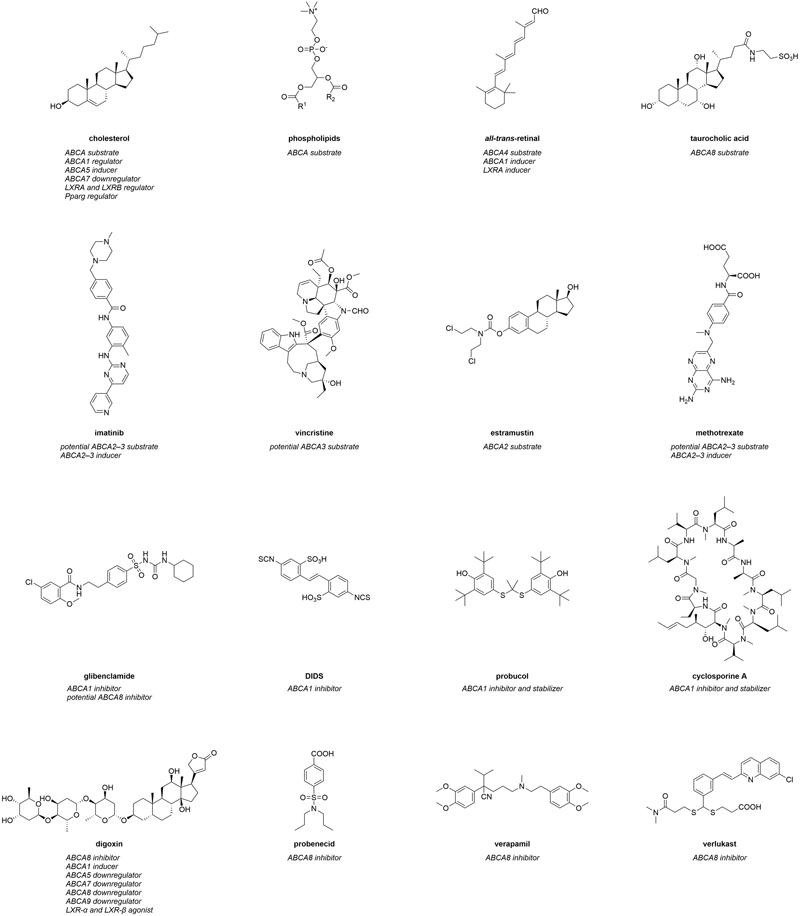
Figure 1. Molecular formulas of prominent interactors of ABCA transporters.
Modulators of ABCA transporter function, trafficking, and regulation
‘Modulation’ is a widely used term to summarize actions of small-molecules that have been reported to alter ABCA transporter function, trafficking, and/or regulation. Modulators can be divided into ‘interactors’ and ‘regulators’.
Interactors summarize compounds that directly bind to ABCA transports, which can have either inhibiting or activating effects on the transporters. Substrates are also included in this category. In terms of ABCA transporters, however, a direct interaction of these agents with their target(s) has in most cases not yet been comprehensively proven. Therefore, compounds that are believed to directly interact with ABCA transporters extend the category of interactors. Figure 1 represents the most prominent interactors of ABCA transporters and provides additional information about their mode of modulation.
Regulators are compounds that change ABCA transporter expression (transcription and/or translation) in terms of induction and/or downregulation. In addition, compounds that regulate ABCA transporter trafficking can be included into the category of regulators, as this effect was often observed as ‘pseudo-protein increase’ at the cell membrane. Figure 2 depicts the most prominent regulators of ABCA transporters including proposed mode of modulations.
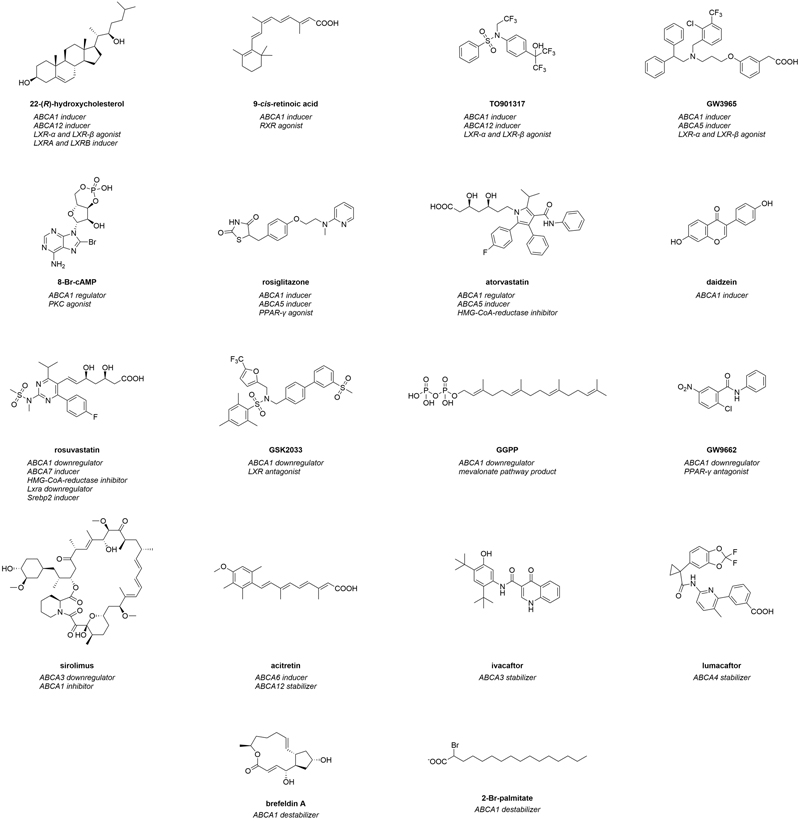
Figure 2. Molecular formulas of prominent regulators of ABCA transporters.
It must be stated that the term ‘inhibitor’ and ‘activator’ are often misused in the literature, as in most cases studies describe a downregulation or induction. In the present review, this mislabeling has been taken into account and the present review and the respective compounds have been allocated into the correct groups. As established earlier,23,24 the compounds are sorted according to their origin: (i) intrinsic substrates and substrate-like molecules, (ii) (other) natural compounds, (iii) pharmacological drugs, (iv) high-throughput screening-(HTS)-derived candidates, as well as (v) compounds from synthetic/medicinal chemistry approaches. Figure 3 gives a general overview of specific interactors and their postulated mode of modulation. Table 2 summarizes all modulators of ABCA1, the most studied ABCA transporter, while Table 3 summarizes all known modulators in terms of the other ABCA transporters. The stated concentration values are indicators of bioactivities of the respective compound and are strongly dependent on the testing system utilized. Hence, the respective data must be interpreted with caution.
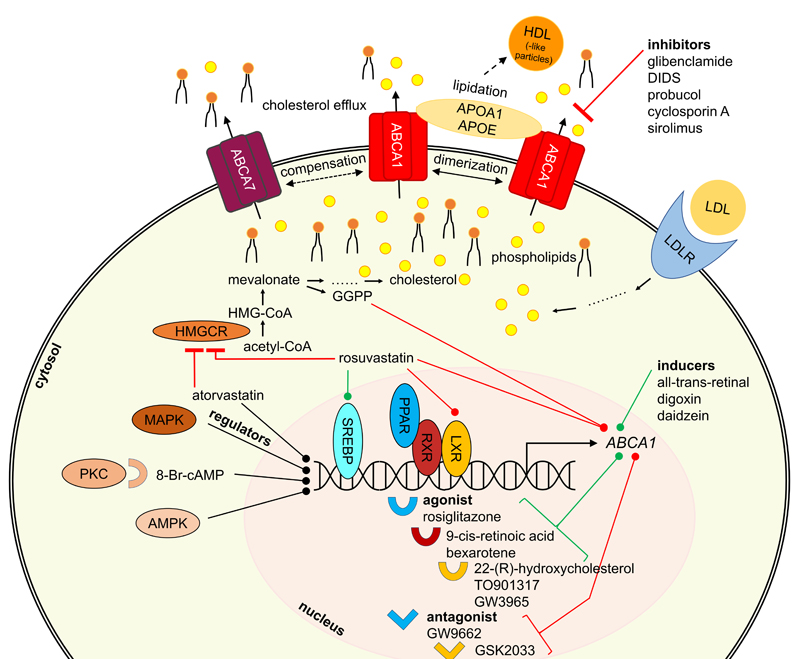
Figure 3. General overview of proteins participating in ABCA1 regulation and interaction.
Small-molecule interactors of ABCA transporters
Endo- and xenobiotic substrates
The most genuine interactors of ABCA transporters are intrinsic substrates of these transporters. These include cholesterol (Figure 1) and other sterol derivatives,10,221,222,228 but also phospholipids (Figure 1), sphingolipids228,229 and retinoids (e.g., all-trans-retinal; Figure 1).133-138 In addition, certain intrinsic molecules were demonstrated to interact with ABCA transporters, in particular with ABCA1230 and ABCA8.10,221,222 α-tocopherol (vitamin E) was demonstrated to be transported by ABCA1,230 and to interfere with ABCA1 regulation.231 The sterol derivatives estradiol-β-glucuronide, estrone sulfate, and taurocholic acid (Figure 1), but also the physiological substrate leukotriene C4 (LTC4), the natural compound ochratoxin A, as well as the chemical p-amino hippuric acid were discovered as (potential) ABCA8 substrates.10,221,222 Specifically the ABCA8-mediated taurocholate export from various human pancreatic cancer cell lines was suggested as the major mechanism behind gemcitabine resistance in these cells,221 which was corroborated in HEK293 cells stably expressing ABCA8.10
In addition, a small body of evidence suggests that ABCA2 and ABCA3 contribute to the subcellular sequestration of certain antineoplastic agents into endo- and lysosomes.232-235 These agents
include cytarabine (ABCA3),235 daunorubicin (ABCA3),232,233,235 etoposide (ABCA3),235 imatinib (ABCA2 and ABCA3; Figure 1),234,236 mitoxantrone (ABCA3),235 and vincristine (ABCA3; Figure 1).235 Furthermore, several antineoplastic agents were described to have less effect when ABCA2 was overexpressed in vitro171,237,238 and in vivo.239 For example, the anticancer drug estramustine (Figure 1) was effluxed from ABCA2-overexpressing human ovary carcinoma cells, which were less susceptible to estramustine treatment than the sensitive cell line.171,238 Antisense nucleotide treatment against ABCA2 re-sensitized the carcinoma cells, further demonstrating a role for ABCA2 in mediating drug efflux.238 Furthermore, Abca2 knock-out mice had elevated estradiol and estrone levels when treated with estramustine.239 A similar effect in terms of susceptibility and re-sensitization was observed for ABCA3-mediated transport of miltefosine in Leishmania,240 doxorubicin resistance in acute myeloid leukemia cells,237 and cisplatin as well as paclitaxel resistance in several lung cancer cell lines.241
| Mode of modulation | Name of modulator | Effect concentration; concentration range; EC50; dose; ED50 |
| (Potential) substrates | cholesterol
phospholipids
β-sitosterol
sphingomyelin
α-tocopherol | -
-
-
-
- |
| Activators | ATI-5261
CS-6253 | 1.07 µM; 30 mg/kg body weight in mice
0.73 µM; 20 mg/kg body weight in mice |
| Inhibitors | BLT-4
bromosulfophthaleine
bumetanide
cyclosporine A
DIDS
diphenylamine 2-carboxylic acid
flufenamic acid
furosemide
glibenclamide
pimecrolimus
probucol
sirolimus
tacrolimus
valspodar | 150 µM
500 µM
200 µM
1–20 µM; IC50 = 5.1–7.6 µM
40–500 µM
500 µM
500 µM
200 µM
50–1000 µM
20 µM; IC50 = 7.0 µM
1.9–20 µM
20 µM; IC50 = 18.8 µM
20 µM; IC50 = 13.6 µM
5 µM; IC50 = 1.9 µM |
| Inducers | A-769662
aclarubicin
allicin
cAMP
butyryl-cAMP
8-Br-cAMP
CPT-cAMP
atorvastatin
ATRA
AZ1–AZ9
AZ-1
AZ-2
AZ10606120
AZ876
BCD1
N-benzothiazolyl-2-benzenesulfonamides
berberine
bergapten
bexarotene
bezafibrate
BMS-852927
sodium-butyrate
cholesterol
cholic acid analog 14b
celastrol
chalcone derivatives
chromene derivatives 2, 3, and 5
chromone analog 6
CL2-57
curcumin
daidzein
danthron
1,6-O,O-diacetylbritannilactone
digoxin
doxazosin
doxorubicin
efatutazone
E3317
EGCG
homo-eriodictyol
ethyl 2,4,6-trihydroxybenzoate
F1
F4
fargesin
fenofibrate
fluvastatin
FPD5
fucosterol
geniposide
ginsenoside (derivatives)
ginsenoside compound K
glycyrrhizine
GQ-11
GW3965
GW7845
gypenosides
hesperetin-7-O-β-D-glucopyranoside
hesperetin-7-O-rutinosid
20-(S)-hydroxycholesterol
4-hydroxycholesterol
22-(R)-hydroxycholesterol
22-(S)-hydroxycholesterol
24-hydroxycholesterol
24-(S)-hydroxycholesterol
25-hydroxycholesterol
27-hydroxycholesterol
3-hydroxytyrosol
idarubicin
kaempferol
L836,978
kuwanon G
L-839,867
LXR623
lycopene
M2
maslinic acid
metformin
mevalonate
mevastatin
mitotane
naringenin
obeticholic acid
ondansetron
orlistat
ouabain
paeonol
PCB29-pQ
pemafibrate
pestalotioquinoside C
phenethyl isothiocyanate
Tadehagi triquetrum-derived glycosides
pioglitazone
pitavastatin
platycodin D
PMA
ponasterone A
pratensein
propofol
prostaglandin J2
pyrrole-imidazole-polyamide
pyrromycin
quercetin
9-cis-retinoic acid
RO0721957/5
RO0264456
rosiglitazone
RPR-5
rutaecarpine and derivatives
saikosaponin A
24-(S)-saringosterol
SB203580
scutellarein
selenium
serdemetan
simvastatin
SPF1
SPF2
soraphene A
24-(S)-stigmast-5-ene-3β,24-diol
Cannabis sativa-derived stilbenoids
sulfoxaflor
tanshindiol C
taraxasterol
testosterone
tetradecylthioacetic acid
TO901317
TR1
trichostatin A
troglitazone
TTNPB
urolithin A
urolithin B
urolithin B sulfate
vitamin D3
vitexin
WAY-254011
Wy14643
bexarotene derivatives Z10 and Z36
zafirlukast | 250 µM
EC50 = 0.49 µM
2.5–10 µM
0.1–10 µM
300 µM
0.3–1000 µM
300–500 µM
5–10 µM; 4 mg/kg body weight in mice
0.25–10 µM
ED50 = 1.49–341 µmol/kg body weight in mice
10 µM
10 µM
10 µM
ED50 = 0.956 µmol/kg body weight in mice
EC50 = 0.035 µM
EC50 = 0.37–33.42 µM
5–20 µM
12.5–50.0 mg/kg body weight in rats
0.1–1 µM
10–200 µM
ED50 = 2.10 µmol/kg body weight in mice
1000–10.000 µM; 200–400 mg/kg body weight in mice
12.9–100 µM
5–40 µM
0.1–1.0 µM; 0.5–1 mg/kg body weight in mice
5–10 µM; 20 mg/kg body weight in mice
25 µM
25 µM
10 µM; 10 mg/kg body weight in mice
5–40 µM
EC50 = 3.17 µM
10–40 µM; 60 mg/kg body weight in mice
8–10 µM; 10 mL/kg body weight in mice
0.010 µM
10 µM
0.0316–1 µM; 20 mg/kg body weight in mice
40 µM
0.01–1 µM; EC50 = 0.2 µM
40 mg/kg body weight in mice
41.4–165 µM
50–100 µM
ED50 = <30 µmol/kg
10 µM
20 µM; 50 mg/kg body weight in mice
2.77–40 µM
1–20 µM
1 µM; 0.005–0.02 mg/kg body weight in mice
100-200 µM
515 µM; 50–100 mg/kg body weight in mice
10–30 µM
1.25 µM
60.8–243 µM
20 mg/kg body weight in mice
0.5–50 µM; ED50 = 0.969 µmol/kg body weight in mice
5 µM
5 µg/mL
107–431 µM
100 µM; 3 mg/kg body weight in mice
5–20 µM
1–20 µM
1–25 µM; EC50 = 1.0 µM
5–20 µM
20 µM
0.5–1.5 µM
2–12.4 µM
6.21 µM–10 µM
2–5 µM
0.1 µM
2.5–10 µM
u.c.a
20 µM
0.1–1 µM
0.1–1 µM; ED50 = 31.5 µmol/kg body weight in mice
2.2–6.6 mg/kg body weight in ferrets
10 µM
20 µM
10 µM
5–500 µM
50 µM
20–50 µM
25–100 µM
40 mg/kg body weight in mice
1 µM
50 µM
0.010 µM
100 µM
5–10 µM
0.1–10 µM; 0.3 mg/kg body weight in mice
50 µM
30–75 mg/kg body weight in mice
10 µM
5–10 µM; EC50 = 1.28–7.474 µM; 20 mg/kg body weight in mice
0.1–10 µM
5–20 µM
0.32 µM
2–5 µM
EC50 = 2.91 µM
50 µM
1–20 µM
1 µM; 1 mg/kg body weight in mice
EC50 = 0.85 µM
20 µM; 12.5 mg/kg body weight in mice
0.04–10 µM; EC50 = 0.29 µM
0.050 µM
0.005 µM
0.05–10 µM; EC50 = 1.49 µM
5 µM
0.035–34.98 µM; EC50 = 0.27 µM
2–8 µM
10 µM
20 µM
50 mg/kg body weight in mice
2.5–5 µM
2–5 µM
10 µM
1 µM
1 µM
0.03–20 µM; EC50 = 0.01391 µM
10 µM
2.5–3 µM
u.d.b in Aphis gossypii
10 µM
3–12 µM
0.001–0.01 µM
0.75% of high-fat diet in mice
0.1–25 µM; ED50 = 4.11 µmol/kg body weight in mice
10 µM
99.2 µM; 0.5 mg/kg body weight in mice
1 µM
0.25–10 µM
20 µM
0.1–10 µM
10 µM
1 µM
50 µM
ED50 = <30 µmol/kg body weight in mice
0.05–100 µM
1 µM; 40 mg/kg body weight in mice
2.5–5 µM |
| Downregulators | 5CPPSS-50
acrolein
8-Br-cAMP
angiotensin II
asymmetric dimethylarginine
atorvastatin
ATR-101
bisphenol A
chalcone derivatives
4-{[4-(4-chlorophenyl)-2-thiazolyl]amino}phenol
cholesterol
dexamethasone
dibutyl phthalate
EGCG
fluvastatin
GGPP
GSK2033
GW6471
GW9662
desulfated holothurin A
homocysteine
lipopolysaccharides
lovastatin
LY294002
methionine
mevalonate
mevastatin
mitotane
NDEA
1,2,3,4,6-penta-O-galloyl-β-D-glucose
phenylalanine-proline
pitavastatin
pravastatin
raloxifene
rosuvastatin
simvastatin
SR9243
tamoxifene
α-tocopherol
γ-tocopherol
toremifene
troglitazone
valproic acid
varenicline | 20 µM
5–20 µM
0.3 µM
0.0001–0.100 µM
0.5–1 µM
0.1–100 µM
10–30 µM
100 µM
10 µM
5 µM
150 µM
0.1–2.5 µM; 8 mg/KG body weight in rats
0.1 µM
100 mg/kg body weight in mice
0.1–100 µM
10 µM–200 µM
0.05–5 µM
10 µM
10 µM
2.68–4.47 µM
50–200 µM
1 mg/mL
0.1–100 µM
20 µM
17 g/kg food in mice
100 µM
0.05–50 µM
50 µM
100 mg/kg body weight in rats
25–300 mg/kg body weight in mice
1000 µM; 600 mg/kg body weight in rats
10 µM
50 µM
10 µM
5–50 µM
0.1–100 µM
1 µM
2.5–10 µM
50–100 µM
50–100 µM
10 µM
10 µM
1000 µM
10 µM; 0.5 mg/kg body weight in mice |
| Stabilizers | cyclosporine A
diphenoquinone
erythrodiol
ALLN
leupeptin
probucol
spiroquinone
testosterone
wogonin | 10 µM
0.0001–0.0005 µM
10–15 µM
50 µM
1170 µM
u.c.a
0.025–0.050 µM
0.01 µM
10–40 µM |
| Destabilizers | brefeldin A
2-bromopalmitate
cycloheximide
Gö6976
monensin A
serdemetan
tunicamycin | 17.8–36 µM
7.5–60 µM; IC50 = 15 µM
355 µM
10 µM
10 µM
2–5 µM
2.41 µM |
a u.c. = unspecified concentration
b u.d. = unspecified dose
Strikingly, ABCA2 co-localized with the lysosomal-associated membrane protein 1 (LAMP1) – an endolysosomal marker – as well as the fluorescence probe dansyl-estramustine. This co-localization indicates a direct sequestration of this antineoplastic drug into endo- and/or lysosomes.171 On the other hand, the susceptibility of ABCA3-overexpressing CCRF-CEM leukemia cells to the antineoplastic agents cytarabine, methotrexate (Figure 1), vincristine, but also the anti-inflammatory drug dexamethasone, was reduced compared to their parental counterparts.242 Taken together, ABCA2 and ABCA3 are contributors to MDR, and the number of potential ABCA2 and ABCA3 substrates may be even higher than currently suggested.
Interestingly, missense mutations of ABCA4 were associated with chloroquine- and hydroxychloroquine-associated retinopathy,243 although contradictory studies exist.244 A direct interaction was postulated, however, not proven. Nevertheless, these results suggest chloroquine and hydroxychloroquine as potential ABCA4 substrates.
| Mode of modulation | Name of modulator | Effect concentration; concentration range; EC50; dose; ED50 |
|
| ABCA2 | | |
| (Potential) substrates | cytarabine
dexamethasone
estramustine
estradiol
estrone
imatinib
methotrexate | -
-
-
-
-
-
- |
| Inducers | imatinib
methotrexate
progesterone
sulfoxaflor
U18666A | u.c.b
1.28 µM
31.8 µM
u.d.c in Aphis gossypii
5 µM |
| Downregulators | celecoxib | 10 µM |
|
| ABCA3 | | |
| (Potential) substrates | cisplatin
cytarabine
dasatinib
daunorubicin
dexamethasone
doxorubicin
etoposide
imatinib
methotrexate
miltefosine
mitoxantrone
nilotinib
paclitaxel
vincristine | -
-
-
-
-
-
-
-
-
-
-
-
-
- |
| Inducers | dasatinib
5-FU
imatinib
methotrexate
nilotinib
vitamin C | u.c.b
50 µM
0.1–12.5 µM
1.28 µM
u.c.b
56.78 µM |
| Downregulators | genistein
indomethacin
lipopolysaccharides
PK11195
sirolimus | 3–9 µM
2 µM
10 µg/mL; 100 µg/mL in chicken lungs
u.c.b
2 µM |
| Stabilizers | C13
C14
C17
genistein
ivacaftor
| 10 µM
10 µM
10 µM
10 µM
1 µM |
|
| ABCA4 | | |
| (Potential) substrates | chloroquine
hydroxychloroquine
β-ionone
11-cis-retinal
13-cis-retinal
all-trans-retinal
all-trans-retinoic acid
all-trans-retinol
N-retinylidene-phosphatidyl-ethanolamine
phosphatidyl-ethanolamine | -
-
-
-
-
-
-
-
-
- |
| Stabilizers | C3
C4
C18
lumacaftor | 10–20 µM
1–20 µM
10–20 µM
10–20 µM |
|
| ABCA5 | | |
| Inducers | atorvastatin
bezafibrate
cholesterol
GW3965
rosiglitazone
tacrolimus
troglitazone | 20 µM
10 µM
100–150 µM
0.5 µM
10 µM
0.04 µM
10 µM |
| Downregulators | digoxin | 2.5 g/kg body weight in mice |
|
| ABCA6 | | |
| Inducers | acitretin
lovastatin
mevastatin | 1–10 mg/kg body weight in pigs
10 µM
10 µM |
| Downregulators | lovastatin
mevastatin | 10 µM
10 µM |
|
| ABCA7 | | |
| Inducers | ponasterone A
pravastatin
rosuvastatin | 1–5 µM
50 µM
5 µM |
| Downregulators | cholesterol
digoxin
25-hydroxycholesterol | 2 mM
2.5 g/kg body weight in mice
2.48 µM |
|
| ABCA8 | | |
| (Potential) substrates | p-aminohippuric acid
estradiol-β-glucuronide
estrone sulfate
glibenclamide
leukotriene C4
ochratoxin A
taurocholic acid | -
-
-
-
-
- |
| (Potential) inhibitors | digoxin
dofequidar
glibenclamide
ochratoxin A
probenecid
verapamil
verlukast | 250 µM
10 µM
250 µM
50 µM
1000 µM
1000 µM
100 µM |
| Inducers | gemcitabine
polyethyleneglycol-block-polyactide nanoparticles | 0.05–0.8 µM
42.04 g/kg body weight in rats |
| Downregulators | digoxin | 2.5 g/kg body weight in mice |
|
| ABCA9 | | |
| Downregulators | digoxin | 2.5 g/kg body weight in mice |
|
| ABCA12 | | |
| Inducers | ceramide N-hexanoyl-D-erythro-sphingosine
ciglitazone
D609 xanthate
D-DDMP
GI 251929X
GW610742
D-MAPP
D-NMAPPD
D-PPMP
D-PPPP
22-(R)-hydroxycholesterol
TO901317
troglitazone | 5 µM
7.5 µM
25 µM
u.c.b
10 µM
8 µM
10 µM
5 µM
5 µM
10 µM
10 µM
10 µM
7.5 µM |
| Stabilizers | acitretin | 1–10 mg/kg body weight in pigs |
a apart from cholesterol and/or phospholipids
b u.c. = unspecified concentration
c u.d. = unspecified dose
Inhibitors
To date, the number of small-molecules that (are believed to) directly interact with ABCA transporters is very low. For example, only 14 inhibitors can be found in the literature regarding the most studied prototype of ABCA transporters, ABCA1.245-248 Only four of these inhibitors are associated with half-maximal inhibition concentrations (IC50),245,249 which is the ‘golden surrogate’ to evaluate and judge inhibitory activities of small-molecules. The following section will highlight these small-molecules as well as inhibitors of other ABCA transporters.
ABCA1
Glibenclamide and 4,4’-diisothiocyano-2,2’-stilbenedisulfonic acid (DIDS)
As outlined above, ABCA1 is the most studied and understood ABCA transporter, although its particular role in neurodegenerative diseases in general51,103 – and in AD in particular – is not well understood.28-30,43,95,102 However, over time, several agents were found to impact ABCA1 transport function. The most prominent examples are glibenclamide and DIDS (both Figure 1), which were first shown to inhibit ABCA1 in 1997.247,248 These drugs blocked the ABCA1-mediated 125I efflux from murine peritoneal macrophages247 as well as human ABCA1-transfected Xenopus laevis Oocytes.248 Glibenclamide and DIDS inhibited the ABCA1-mediated transport of cholesterol and other sterols as well as phospho- and sphingolipids. Thus, these agents became the ‘standard ABCA1 inhibitors’ and have frequently been used in ABCA1 studies ever since.229,250-269 Glibenclamide and DIDS were preferred over other discovered ABCA inhibitors, such as bumetanide, diphenylamine 2-carboxylic acid, flufenamic acid, furosemide, and bromosulfophthaleine.248 Specifically glibenclamide was rigorously evaluated regarding its mechanism of action. It was demonstrated that glibenclamide prevented cross-linking of 125I-marked APOA1 to ABCA1,267,270 not interfering with ABCA1 location at the cell surface.267 In essence, glibenclamide and DIDS may play a significant role in the development of future modulators of ABCA transporters in general.
Probucol and cyclosporine A
Less prominent but also well characterized are the antilipidemic drug probucol246,271-278 and the immunosuppressant cyclosporine A245,249,258,279-281 (both Figure 1). Probucol was demonstrated to reduce the cholesterol efflux from different
ABCA1-overexpressing murine and human
macrophages,275-278 and total lipid release (cholesterol + phospholipids) from human WI-38 fibroblasts.246 Vice versa, probucol increased accumulation of free cholesterol, cholesterol esters, phosphatidylcholine, and sphingomyelin in human fibroblasts.246 Additionally, probucol was reported to prevent cell surface-specific binding of 125I marked APOA1 to ABCA1.246,278 Similarly, this effect has already been demonstrated for glibenclamide before.267,270 Interestingly, it was shown that total ABCA1 protein levels were increased after exposure to probucol due to decreased degradation.246,275 This qualifies probucol also as a stabilizer. However, as its inhibiting effect is far more pronounced, we have included it as an inhibitor here.
The immunosuppressant cyclosporine A has been characterized as an ABCA1 inhibitor in multiple studies.245,249,258,279-281 This inhibition was shown to be direct through a radiolabeled variant of cyclosporine A and purified ABCA1.245 Cyclosporine A not only functionally inhibited ABCA1-mediated cholesterol and phospholipid efflux,245,249 and caused intracellular accumulation of cholesterol,258 but also inhibited the ABCA1-dependent binding of Alexa 546- or 125I-labeled APOA1,245,249 as demonstrated for glibenclamide267,270 and probucol246,278 before. Interestingly, toxicity assays demonstrated that cyclosporine A negated the positive effect of an ABCA1 inducer on cell viability when cells were exposed to Aβ proteins.280 This was confirmed in vivo in C57BL/6 mice that had reduced HDL levels.249 Interestingly, cyclosporine A was shown to decrease ABCA1 turnover, increasing its presence at the cell surface by a factor of two as demonstrated with a GFP-labeled ABCA1 variant,249 suggesting a similar mode of inhibition as for probucol.275 Thus, as for probucol,246,275 cyclosporine A also appears to have a stabilizer function,275 but is included in the current section due to its pronounced inhibitory role. Morevover, the cyclosporine A analog valspodar (PSC833) inhibited direct binding of radiolabeled cyclosporine A to ABCA1, revealing that valspodar also acts as an ABCA1 inhibitor.245,282 Furthermore, several other calmodulin antagonists inhibited ABCA1-mediated cholesterol efflux and binding of APOA1.245 These include pimecrolimus,245 sirolimus,245 and tacrolimus,245 suggesting these molecules as potential scaffolds for the development of future ABCA1 modulators.
Other ABCA1 inhibitors
In terms of other small-molecules that were suggested to inhibit ABCA1 function, BLT-4 has been demonstrated to inhibit cholesterol and phospholipid export from adipocytes and macrophages,255 and to decrease cholesterol efflux from ABCA1-transfected HEK293 cells. BLT-4 was also shown to inhibit 125I-marked APOA1-binding to ABCA1,270 as demonstrated for glibenclamide,267,270 probucol,246,278 and cyclosporine.245,249
Other ABCA transporters While ABCA1 can be considered a less-studied ABC transporter with certain knowledge about its function and interfering small-molecules,18 all other ABCA transporters belong to the group of under-studied ABC transporters that cannot be addressed by small-molecules with very rare exceptions.18
One rare example is ABCA8. Using the Xenopus laevis Oocytes model in vitro testing system,248 Tsuruoka et al. reported inhibitors of this transport protein.222 While digoxin, probenecid, and verapamil (all Figure 1) could be identified as very weak inhibitors of ABCA8-mediated estradiol-β-glucuronide transport, dofequidar (MS-209), ochratoxin A, and verlukast (MK-571; Figure 1) were discovered as moderately potent inhibitors.222 In addition, glibenclamide was also suggested to (partially) inhibit ABCA8 function.266
Activators Although activators of ABC transporters have been reported, as for example, for ABCB123 and ABCC transporters,23,283-288 these reports are somewhat scarce compared with other classified modulators of ABC transporters. In terms of A subclass ABC transporters, no small-molecule activators are known. However, it is well established and has been extensively demonstrated that ABCA1 activity depends on (co)-administration of HDL and/or APOA1.117 HDL and APOA1 are not small-molecules but peptides, and therefore fall outside of the scope of the present review. Similarly, it has been shown in several reports that HDL-mimics consisting of 26 amino acids are able to increase ABCA1-mediated transport.289 Although these molecules are also not small-molecules, the scarceness of activators of ABCA transporters warrants the inclusion of these middle-sized molecules here.
In 2004, structural elements of APOA1 were discovered to promote ABCA1-mediated cholesterol efflux.290 In 2007, Vedhachalam et al. discovered that the C-terminus of APOE promoted ABCA1-mediated efflux from murine J774.A1 macrophages.291 The latter discovery led to the development of two short-length peptides, ATI-5261 and CS-6253, consisting of 26 amino acids each.289 Their amino acid sequences expressed in single-letter code are EVRSKLEEWFAAFREFAEEFLARLKS289 and EVCitSKLEEWLAALCitELAEELLACit-LKS (Cit = citrulline),292 respectively, which is of particular interest for the development of novel lead structures. Both peptides increased ABCA1-mediated cholesterol and phospholipid transport in murine and human macrophages.289,292 Interestingly, CS-6253 decreased 125I-labed APOA1 binding to ABCA1,292 as demonstrated for glibenclamide,267,270 probucol,246,278 cyclosporine A,245,249 and BLT-4270 before. However, CS-6253 was shown to compete with APOA1 to promote ABCA1-mediated transport.292 Both ATI-5261 and CS-6253 have a high practical relevance regarding AD and other neurodegenerative diseases, as these agents demonstrated in vivo efficacy.289,293 ATI-5261 treatment of high fat diet-fed Apoe knock-out mice decreased cholesterol levels in both plasma and feces and reduced atherosclerotic lesions.289 For CS-6253, a reduction of Aβ42 levels and tau protein phosphorylation in transgenic humanized APOE4 mice was demonstrated, which was accompanied by improved cognitive functions.293 Interestingly, an elevation of ABCA1 protein was also observed in treated mice.293 Indeed, a stabilization and/or induction may also have contributed to the observed effects. However, the proven direct binding of these agents suggested that activation takes place as the major mode of action. Nonetheless, CS-6253 has not been tested in AD mouse models so far, and being a peptide, it would not be suitable for oral application in patients.
Small-molecule regulators of ABCA transporters
The herein discussed regulators interfere with ABCA transporter expression and/or trafficking. Important representatives are depicted in Figure 2 and additional information is given in terms of their mode of modulation. Since many different pathways are involved in ABCA transporter regulation, Figure 3 provides a general overview of participating proteins and protein families in terms of the most studied ABCA transporter, ABCA1.
Inducers ABCA1 - LXR and RXR pathways Given the findings in AD mouse models with knock-out of ABCA1/Abca1 or overexpression of ABCA1, upregulating ABCA1 activity may be a therapeutic strategy for decreasing Aβ pathology in AD. ABCA1 is under the transcriptional control of the nuclear receptors liver-X-receptor (LXR) and retinoid-X-receptor (RXR),294-296 which can be targeted by small-molecule agonists of LXR and RXR to induce ABCA1 expression (Figure 3). Numerous studies reported that treatment of APP-transgenic mice with LXR or RXR agonists decreased Aβ load126,297-301 and/or improved cognitive impairment.126,297,298,300 Other studies reported cognitive improvement without significant changes in Aβ load in APP-transgenic mice treated with LXR agonists.302,303 LXR and RXR agonists have already been described extensively as potential therapeutics in the literature, also with respect to AD.304 The present review will focus on those agonists that were reported in clear association with ABCA1.
Oxysterols and retinoic acids 22-(R)-hydroxycholesterol (Figure 2) has been established as the natural gold standard for ABCA1/Abca1 induction through LXR activation,122,205,249,252,259,262-264,268,277,278,305-315 while 9-cis retinoic acid (Figure 2) became the natural gold standard for RXR activation.122,245,249,259,262,264,277,278,309,311,313,316 The inducing effects were described both on ABCA1/Abca1 mRNA122,205,252,263,264,305,307-311,313,315-317 and ABCA1 protein levels.122,252,263,264,306,309-311,316,318
Other oxysterols like 4-hydroxycholesterol, 20-(S)-hydroxycholesterol, 22-(S)-hydroxycholesterol, 24-hydroxycholesterol, 24-(S)-hydroxycholesterol, 25-hydroxycholesterol, 27-hydroxycholesterol, and cholesterol itself also induced ABCA1/Abca1 mRNA205,305,313,315,319-327 and ABCA1 protein levels.321,328 The increase in ABCA1 protein was functionally confirmed by an enhanced cholesterol305,306,313,315,318 and phospholipid efflux,311,318 as well as reduced total cholesterol influx.305 Specifically 22-(R)-hydroxycholesterol and cholesterol induced both LXRA/Lxra and LXRB/Lxrb.310,321 Additionally, cholesterol also induced murine peroxisome proliferator-activated receptor γ (PPAR-γ) mRNA (Pparg),321 which represents an important alternative pathway for ABCA1/Abca1 induction. Furthermore, 24-(S)-hydroxycholesterol reduced in parallel the sterol regulation element-binding protein 2 (SREBP2) gene expression (Srebp2).323 The SREB protein family also represents another important pathway in ABCA1/Abca1 regulation.
The 9-cis-retinoic acid derivative all-trans¬-retinoic acid (ATRA) significantly increased ABCA1/Abca1 mRNA and ABCA1 protein content in murine and human macrophages, which was paralleled by increased LXRA mRNA levels in human macrophages.329 This increase resulted in a subsequently enhanced cholesterol efflux from murine macrophages. ATRA is an agonist of the retinoic acid receptor (RAR),329 which is in close relation to the RXR receptor and a potential target of retinoic acid derivatives.
TO901317 and GW3965 The synthetic gold standard and most studied ABCA1/Abca1 inducer in the literature is TO901317 (often referred to as ‘T0901317’; Figure 2).205,245,250,252,259,260,262,264,271,272,279,280,282,308,310,317,319,322,324,326,328-345 TO901317 targeted both the LXR α250,310,328,330,332,335,337-340,342 and LXR β pathways,250,310,338,342 which correlated to ABCA1/Abca1 induction on mRNA and ABCA1 protein levels.205,250,279,282,310,319,322,324,326,328,330-335,337-340,342,343 In addition, an induction of SREBP1C/Srebp1c has also been observed.336,342 Functionally, TO901317 increased cholesterol efflux,250,259,260,262,264,282,319,324,329,331,342 decreased intracellular Aβ content, and increased Aβ secretion from different murine brain cells.126,345 Further, it reduced Aβ25-35-mediated toxicity toward cells by induction of Abca1.280 In addition, TO901317 mitigated memory deficits in high-fat diet-fed APP23 mice, reducing both plaque and soluble Aβ protein levels.344 Besides, TO901317 reduced methionine-(homocysteine)-induced atherosclerotic lesions in Apoe knock-out C57BL/6 mice.335 These findings were paralleled by an increase of Abca1 mRNA and ABCA1 protein content,335 suggesting a potential relevance of TO901217 in AD therapy, although it must be taken into account that LXR activators, in particular TO901317, were demonstrated to have severe side effects in mice, such as neutropenia, hypertriacylglycerolemia, hepatic triacylglycerol accumulation, and hepatic steatosis.271,346,347
The second most common synthetic
LXR-α and LXR-β agonist is GW3965 (Figure 2).255,272,317,319,321,334,348-352 GW3965 increased mRNA317,319,321,348,349,351,352 and protein levels255,272,351 in different ABCA1-expressing cells. Functionally, increased Abca1 mRNA and ABCA1 protein levels correlated with enhanced cholesterol efflux.255,351 Strikingly, exposure of murine BV2 microglia to GW3965 reduced Aβ42 levels due to an enhanced degradation of Aβ42,126 suggesting that ABCA1 contributes to general Aβ degradation. Finally, GW3965 significantly increased Abca1 transcription in C57BL/6 mice,334,351 and improved contextual memory as well as Aβ pathology in TG2576 mice,126 emphasizing its high relevance in AD therapy.
ABCA1 - other LXR agonists and inducers Sterane and sterane-like natural compounds Several sterane derivatives were demonstrated to target LXR-α and LXR-β activation253,307,310,353 and/or LXRa/Lxra and LXRB/Lxrb upregulation,330,332,354,355,356,357 resulting in induction of ABCA1/Abca1. Celastrol,330,332 digoxin,253 fuco-sterol,308 certain gypenosides,354 ouabain,253 platy-codin D,355 saikosaponin A,356 24-(S)-saringosterol,307 24-(S)-stigmast-5-ene-3β,24-diol,307 taxarasterol,353 testosterone,357 and TR1310 increased ABCA1/Abca1 mRNA307,308,310,330,332,353,354,356,357 and/or ABCA1 protein content310,253,353,354,355,357 leading to an enhanced efflux of cholesterol in vitro253,308,330,332 and decreased intracellular cholesterol and/or phospholipid levels in vitro330,332,354,356,357 and in vivo in mice.253 The effect of fucosterol was comparable to that of the standard ABCA1/Abca1 inducer TO901317.308 A correlation to SREBP1(C) upregulation308,307,357 and SREBP1 protein expression357 could be determined in case of fucosterol,308 24-(S)-saringosterol,307 24-(S)-stigmast-5-ene-3β,24-diol,307 and testosterone.357 In case of celastrol, the regulation of intracellular cholesterol was pinned to an activation of autophagy330,332 and lipophagy,330 which are processes that may be associated with Aβ degradation.
Flavonoids The flavonoids naringenin,339 quercetin,358 and vitexin359 increased ABCA1/Abca1 mRNA339,359 and ABCA1 protein levels339,360,358 by induction of LXRA/Lxra mRNA358,359 and LXR-α protein.339,360 The effect of naringenin and the standard ABCA1/Abca1 inducer TO901317 were additive. Naringenin was shown to be dependent on the cAMP-activated protein kinase (AMPK) regulation (AMPK), as well as SREBP1C regulation.339 The AMPK pathway is another very important regulator of ABCA1 expression. Functionally, cholesterol efflux from human339,360 and murine360 macrophages was increased in the presence of naringenin.339,360 In vivo, naringenin and quercetin induced Abca1360 and ABCA1,361,362 as well as ABCA1-mediated cholesterol transport,360 which was reflected in reduced atherosclerotic lesions in the aorta of high-fat diet-fed C57BL/6 mice.360 In terms of quercetin, a protein increase of LXR-α and PPAR-γ was observed.361
Chalcones, the precursors of flavonoid biosynthesis, were also demonstrated to intervene with ABCA1 expression. The chalcone derivatives 1h,363 1m,363,364 and 1m-6364 were demonstrated to increase ABCA1 mRNA and ABCA1 protein levels in THP-1 macrophages,363,364 which was accompanied by an increase in LXRA mRNA and LXR-α protein levels.363 The intracellular lipid content was decreased, while the cholesterol efflux was increased after exposure of THP1-cells to 1m-6.364 In addition, SREBP1 mRNA was increased by 1m-6,364 and aortic atherosclerotic plaques were reduced in Ldlr knock-out C57BL/6 mice.364
Polyphenols and diterpenoid natural compounds The polyphenols kuwanon G,365 paeonol,252 the Celtis biondii-derived compound ethyl 2,4,6-trihydroxybenzoate,342 and the diterpenoid farnesin366 increased ABCA1/Abca1 mRNA252,342,365,366 and ABCA1 protein252,342,365,366 content in an LXR-α-252,366 and LXR-β-dependent342 manner, which in parallel reduced cholesterol content252 and increased ABCA1-mediated cholesterol efflux in various cell lines.252,342,366 In vivo, farnesin increased ABCA1 protein content and cholesterol efflux in Apoe knock-out C57BL/6 mice in primary peritoneal macrophages and the aorta, which was reflected in reduced atherosclerotic plaques.366
Other natural compounds Several other natural compounds induced ABCA1/Abca1 targeting LXR-α and LXR-β activation256,272,256,349,367 and/or LXRA/Lxra and LXRB/Lxrb induction.331,348,350,368,369,370,371,372,373,374 The garlic ingredient allicin,350 the alkaloid berberine,256 the coumarin bergapten A,368 certain Pestalotiopsis neglecta-derived chromene derivatives,348 the Rheum palmatum-derived anthra-quinone danthron,369 the lacton 1,6-O,O-diacetylbritannilactone,371 epigallocatechin gallate (EGCG),370 the glycoside geniposide,375 the vegetable ingredient phenethyl isothiocyanate,373 the carotenoid lycopene,372 the Pestalotiopsis neglecta-derived hydroquinone pestalotioquinoside C,349 the alkaloid rutaecarpine,367 selenium,374 the macro-lactone soraphene A,272 and vitamin D3331 led to increased ABCA1/Abca1 mRNA256,272,331,348,369,256,367,370,372,373 and ABCA1 protein256,272,331,349,350,368,369,256,367,371,373,374 content in vitro331,349,350,369,375,374 and in vivo,368,369,370,371,372,373 enhancing cellular cholesterol efflux256,272,256,367,369 and reducing intracellular cholesterol con-tent.331,350,369,256,367,375,372,374 Danthron also increased AMPK protein levels,369 while EGCG downregulated Srebp1 mRNA and SREBP1 protein content.370 Lycopene induced Ppara mRNA in tobacco carcinogen- and cigarette smoke-exposed ferrets,372 while isothiocyanate induced Pparg mRNA as well as PPAR-γ protein content in high fat diet-fed C57BL/6 mice.373 The inducing effects on ABCA1 expression of vitamin D3 and TO901317 were additive.331 Danthron, EGCG, geniposide, and rutaecarpine demonstrated also reduced atherosclerotic lesions in Apoe knock-out C57BL/6 mice,369,370,375,367 and isothiocyanate ameliorated the aortic injury of the high-fat diet in the same mice.373
Pharmacological drugs Several pharmacological drugs also demonstrated an induction of ABCA1/Abca1 through LXR-α and/or LXR-β, including the α1-blocker doxazosin,376 the 5-HT3 receptor antagonist ondansetron,279 and the anesthetic propofol.377 Consequently, increased Abca1 mRNA279,376 and ABCA1 protein279,376 levels were observed in human279,377 and murine279,376 macrophages376,377 as well as astrocytes.279 Functionally, ondansetron induced APOE efflux,279 while propofol led to increased cholesterol efflux.377 In addition, propofol increased PPARG mRNA and PPAR-γ protein content in human macrophages.377
Furthermore, certain antineoplastic agents interfered with ABCA1 expression via LXR-α and/or LXR-β. Doxorubicin demonstrated an Lxr activation with subsequent induction of Abca1 mRNA and ABCA1 protein in vitro and in vivo.250 Functionally, doxorubicin elevated cholesterol export in vitro. It was shown that intra- and extracellular levels of cholesterol, cholesterol precursors, and several oxysterols were elevated after exposure to doxorubicin. These precursors included lathosterol, lanosterol, and desmosterol, while the oxysterols included 7-α-hydroxycholesterol, 7-β-hydroxycholesterol, 7-ketocholesterol, 24-hydroxycholesterol, and 27-hydroxycholesterol. The authors suggested that doxorubicin exposure induced cholesterol metabolism subsequently leading to an induction of ABCA1. Besides, idarubicin augmented also Abca1 mRNA levels in vitro.
Synthetic compounds and HTS hits Other synthetic compounds have been shown to induce ABCA1/Abca1 expression by LXR-α and/or LXR-β induction. The polymer pyrrole-imidazole-polyamide activated a promoter region for Abca1 expression and thereby increased cholesterol and lipid efflux from RAW264.7 cells.376 The authors confirmed their findings in vivo, revealing increased Abca1 mRNA and ABCA1 protein content in peripheral blood mononuclear cells and the liver in C57BL/6 mice after exposure to pyrrole-imidazole-polyamide.
In addition, the LXR agonist LXR623 induced ABCA1 mRNA and ABCA1 protein levels in two human renal adenocarcinoma cell lines334 as well as Abca1 mRNA levels in vivo in C57BL/6 mice.378 This induction was reflected in reduced intracellular cholesterol and triglyceride levels.
It must be noted that several other synthetic LXR-α and LXR-β agonists induced Abca1 expression in vivo: AZ1–AZ9, AZ876, BMS-852927, F1, WAY254011.378 Finally, an HTS approach discovered two LXR-α and LXR-β agonists as novel small-molecule ABCA1/Abca1 inducers: F4 and M2.319
Synthetic approaches A few synthetic approaches have aimed toward the development of ABCA1/Abca1 inducers.271,336,352,379-382 The cholic acid analog 14b,336 the thiophene derivative CL2-57,271 as well as derivatives of N-benzothiazolyl-2-benzene-sulfonamide,379 ginsenoside,352 and rutaecarpine,367 all induced ABCA1/Abca1 mRNA336,352,381 and ABCA1 protein271,336,379,381 content in vitro271,336,379 and in vivo,271 targeting the LXR-α/LXR-β pathway352 by activation271 or induction336 of LXR-α/LXRA/Lxra and/or LXR-β/LXRB/Lxrb. In vitro, cholesterol efflux increased379,381 and intracellular cholesterol as well as lipid content were reduced,336,352 while plasma and liver triglycerides levels were reduced in vivo in high fat diet-fed C57BL/6 mice.271 Interestingly, 14b induced farnesoid-X-receptor (FXR) transcription (Fxr),336 and CL2-57 inhibited RXR-β, PPAR-γ, and PPAR-δ,271
Finally, Singh et al. described highly potent LXR-α and LXR-β agonists with effect at concentrations in the nanomolar range.382 The described podocarpic acid derivatives have not yet been demonstrated to induce ABCA1. However, these compounds were designated as potential ABCA1 inducers by the authors,382 and their high potency makes them interesting candidates for further evaluation.
Such synthetic approaches should be highlighted,271,336,352,379-382 as chemical derivatization of ABCA1 inducers and elucidation of their structure-activity relationships (SAR) have not yet been comprehensively assessed. More reports are needed to gain innovative molecules that can be considered clinically for the treatment of various ABCA1-related diseases.
ABCA1 - other RXR agonists and inducers In terms of synthetic RXR agonists, the 4-chromanon derivatives SPF1 and SPF2 increased Abcb1 mRNA and ABCA1 protein levels and lowered Aβ25–35-mediated cell toxicity in vitro.280 The same effect was observed for the RXR agonist bexarotene,280 an FDA approved drug against T-cell lymphoma-related cutaneous malformations. Bexarotene was used as a standard inducer of ABCA1/Abca1 via the RXR pathway in several studies.271,272,280,319,380 Induction of Abca1 mRNA and ABCA1 protein levels was maximal for bexarotene in combination with TO901317.280 Bexarotene is of particular practical relevance as a potential treatment against AD due to its in vivo effects. In different AD mouse models, bexarotene increased Abca1 mRNA and ABCA1 protein levels, but also reduced cerebral load of Aβ and hyperphosphorylated protein tau, which is also a histological marker in AD and other dementias.297,383 This prospect led to synthetic bexarotene derivatives, specifically Z10 and Z36.380 Both candidates induced ABCA1 protein expression by RXR-α activation and reduced Aβ burden in the hippocampus of female APP/PS1 mice. This coincided with an enhanced ABCA1 protein expression in BV2 cells.
Moreover, the pan-RAR agonist TTNPB also increased ABCA1 protein content in murine macrophages in an RXR-α-dependent manner. However, the effect was generally smaller compared to the effect of ATRA.329 Finally, a combination of the LXR and RXR agonists RO0721957 and RO0264456 increased ABCA1 mRNA in THP-1 macrophages accompanied by increased cholesterol efflux.384 RO0264456 was demonstrated to increase ABCA1 protein content in combination with TO901317.260
ABCA1 – protein kinase C (PKC), AMPK, and p38 mitogen-activated protein kinase (MAPK) An alternative approach to induce ABCA1 is targeting the PKC pathway (Figure 3). PKC agonists were extensively used to induce ABCA1/Abca1 mRNA and ABCA1 protein levels.230,248,249,255,265,266,273,278,289-292,384-387 Prominent PKC agonists include cAMP313 as well as synthetic derivatives, such as 8-Bromo-cAMP (8-Br-cAMP; Figure 2),230,249,255,266,290,292 8-(4-chlorophenylthio)-cAMP (CPT-cAMP),273,291,384 and dibutyryl-cAMP.385-387 The observed effects ranged in the same order of magnitude as the combination of 22-(R)-hydroxycholesterol and 9-cis-retoic acid.313 The increase in ABCA1/Abca1 mRNA and ABCA1 protein levels was reflected in an enhancement of ABCA1-mediated cholesterol and phospholipid efflux,249,255,386 and increased APOA1 binding to murine RAW264.7 macrophages.385-387 Similar observations have been made for the PKC stimulant phorbol 12-myristate 13-acetate (PMA), which induced ABCA1 protein expression and ABCA1-mediated cholesterol and phospholipid release.386 PMA is also the standard substance used to differentiate human monocytic leukemia cells into THP-1 macrophages –
a standard host system for ABCA transporter evaluation.231,245,249,256,268,272,275,292,308,310,312-316,321,328,335,338,
339,341,342,360,363,364,366,377,384,388-397
Regarding the AMPK pathway (Figure 3), the natural compound curcumin induced ABCA1/Abca1 mRNA338,388 and ABCA1 protein levels388,394 as well as cholesterol efflux338,388,394 in THP-1338,388,394 and RAW264.7394 macrophages, which was also mediated through LXR-α activation.338 However, these LXR-α activating effects were much more pronounced in combination with the gold standard TO901317.338 Other AMPK-targeting agents are A 769662 and metformin,398 which induced ABCA1/Abca1,398 LXRA/Lxra,396,398 and LXRB/ Lxrb396,398 in human398 and murine (primary) macrophages,398 leading to increased cholesterol efflux.396
Concerning the MAPK pathway (Figure 3), the sterane glycoside ginsenoside compound K increased Abca1 mRNA and ABCA1 protein levels in murine macrophages, reducing intracellular lipid content and promoting autophagy.399 These effects were pinned to a negative impact on the MAPK pathway. Finally, a synthetic inhibitor of MAPK, SB203580, was shown to induce ABCA1 protein in combination with the above mentioned geniposide in vitro in murine macrophages.375
ABCA1 - the PPAR Pathway Another well-known approach to induce ABCA1 involves the PPAR pathway (Figure 3).268,272,295,309,315,321,326,
327,337,343,395,400-409 Certain PPAR/Ppar inducers and/or PPAR activators have been described above, as these modulators also have effects on the LXR pathway.321,361,372,373,377
Several natural compounds target the PPAR pathway, such as the flavonoids homo-eriodictyol,402 hesperetin-7-O-β-D-glucopyrano-side,402 scutellarein,403 and the antimycotic trichostatin A.410 These compounds increased Abca1402 and Pparg402 mRNA as well as ABCA1,402,410 PPAR-α,403 and PPAR-γ402,410 protein levels in vitro402,410 and in vivo.403 Decreased intracellular cholesterol levels were also observed.402 Trichostatin A reduced aortic atherosclerotic plaques in high-fat diet-fed Apoe knock-out mice,410 and an upregulation of ABCA1, PPAR-γ, and LXR-α/β protein levels was observed in aortic cells as well as peritoneal macrophages.410
Several drugs and drug-like PPAR agonists were revealed to induce ABCA1/Abca1 mRNA and/or ABCA1 protein content, including the PPAR-α agonists fenofibrate,326,400,404 pemafibrate (K 877),405 Wy14643,268,343 and RPR-5,268 as well as the PPAR-γ agonists efatutazone,337 pioglitazone,272,309,326,395,407 pitavastatin,343 prostaglandin J2 (PG-J2),268,327 rosiglitazone (Figure 2),268,309,315,408,409 troglitazone,268 and GW7845,315 but also the broad-spectrum PPAR-α, PPAR-β, and PPAR-γ agonist bezafibrate268,327 and the multitarget PPAR-α, PPAR-γ, and PPAR-δ agonist tetradecylthioacetic acid.401 This induction was observed for ABCA1/Abca1 mRNA268,315,343,401,405 as well as ABCA1 protein levels,268,337,343,395,405,409 and was functionally confirmed by increased cholesterol efflux.268,315 A connection between the PPAR and LXR pathways has also been drawn,268,326,327,337,400 highlighting the importance of both pathways for ABCA1/Abca1 induction. Furthermore, fenofibrate had a positive impact on both the LXR-α and AMPK pathways400 Certain PPAR agonists have been used as standard inducers of Abca1, e.g., pioglitazone407 and rosiglitazone.408
Synthetic PPAR agonists were also reported to induce ABCA1.406 The benzothiazole derivative E3317 dose-dependently increased ABCA1/Abca1 mRNA and ABCA1 protein levels though PPAR-γ activation in several cell lines.406 This was reflected in decreased cholesterol efflux and reduced intracellular cholesterol content. Finally, a molecular docking approach to discover novel PPAR agonists has yielded GQ-11, which induced Abca1 mRNA in livers of C57BL/6 Ldlr knock-out mice.407
ABCA1 - the 3-hydroxyl-3-methyl glutaryl-(HMG)-CoA-reductase pathway Other targets for ABCA1/Abca1 induction are the 3-hydroxyl-3-methylglutaryl-(HMG)-CoA-reduc-tase and cellular cholesterol synthesis (Figure 3).318,343 Several HMG-CoA-reductase inhibitors such as atorvastatin (Figure 2),330,343,362 fluvastatin,312,411 mevastatin (compactin),318 pitavastatin,318,343 and simvastatin312,343 increased ABCA1/Abca1 mRNA312,343 and ABCA1 protein levels,362,411 as well as ABCA1-mediated cholesterol efflux.318 These data are surprising, as one might expect the loss-of-function of an enzyme in the cholesterol synthesis pathway to induce a decrease of ABCA1, preventing cholesterol depletion from cells.314384,412 Conversely, the overproduction of cholesterol leads to the opposite effect, as demonstrated for mevalonate, which is a building block of cholesterol synthesis413 and has been demonstrated to increase ABCA1/Abca1 mRNA312,314 and to abrogate Abca1 downregulation.312 Pitavastatin addressed SREBP-driven promotor regions upregulating Abca1 mRNA levels,343 and atorvastatin reduced atherosclerotic plaques in Apoe knocked-out C57BL/6 mice by induction of ABCA1 protein content in the murine aorta.362
Other ABCA1 inducers Sterane and sterane-like natural compounds Several other agents were reported to induce ABCA1/Abca1 mRNA and/or ABCA1 protein level(s), with some studies reporting a unique mechanism of action for these agents. Such compounds include the sterane derivative ponasterone A (ecdysone; ABCA1 protein; ABCA1-mediated cholesterol and phospholipid transport),202 and the enoxolone derivative glycyrrhizine (ABCA1 protein).414 In addition, the sterane derivative and farnesoid-X-receptor (FXR) activator obeticholic acid induced Abca1 mRNA levels in vivo in the ileum of Srb1-deficient C57BL/6 mice.415 In THP-1 macrophages, the sterane-like maslinic acid induced ABCA1 mRNA levels, paralleled with an increased cholesterol efflux from these cells.390 Finally, the Salvia miltiorrhiza-derived tanshindiol C was demonstrated to induce peroxiredoxin 1 mRNA (Prdx1) and protein (PRDX1) content in murine RAW264.7 cells.416 Prdx1 was demonstrated to regulate Abca1 mRNA and ABCA1 protein expression. A reduction of intracellular cholesterol levels in murine peritoneal macrophages could also be observed.
Flavonoids The flavonoids daidzein (Figure 2),309 kaempferol,397 and pratensein309 induced ABCA1 mRNA309,397 and ABCA1 protein levels309 as well as ABCA1-mediated cholesterol efflux.397In addition, hesperetin-7-O-rutinosid (hesperidin) abrogated the negative effect of varenicline on ABCA1 protein expression in RAW264.7 macrophages.417 The authors could underpin their findings with a reduction of aortic atherosclerotic plaques in Apoe knock-out C57BL/6 mice along with reduced lipid levels in peritoneal macrophages derived from these mice.
Polyphenols and polyphenol-like natural compounds Several polyphenols and polyphenol-like compounds induced Abca1 mRNA408,418 and ABCA1 protein393,404 levels in murine393,404,408,418 and human393 macrophages, leading to an increased cholesterol efflux.404,408,418 These include certain Cannabis sativa-derived stilbenoids404 as well as the Tadehagi triquetrum-derived phenylpropanoid glycosides urolithin A418 and urolithin B (sulfate).393 In vivo, atherosclerotic plaques were reduced after urolothin B treatment. One phenylpropanoid glycoside was demonstrated to increase Lxra, but none of the other compounds could confirm these results. Given that the effect of all compounds on ABCA1 expression was similar, it is likely that another, yet unknown pathway was the major contributor to the observed effects.
Other natural compounds Sodium butyrate induced Abca1 mRNA and ABCA1 protein levels in murine RAW264.7 cells, accompanied by an increased efflux of cholesterol from these cells.419 This induction was reflected by increased ABCA1 protein content in vivo, reduced plasma cholesterol and triglyceride levels, and reduced aortic atherosclerotic lesions and hepatic steatosis in high fat diet-fed Apoe knock-out C57BL/6 mice.
Pharmacological drugs Several pharmacological drugs induced ABCB1/Abca1 mRNA309,420,421 and ABCA1 protein,309,391,421 including the anti-obesity drug orlistat,391 the antibiotic sulfoxaflor,420 the leukotriene receptor antagonist zafirlukast,421 as well as the anthracyclines aclarubicin309 and pyrromycin.309 Zafirlukast in particular reduced intracellular cholesterol and lipid content in oxidized LDL-(oxLDL)-induced lipid-overloaded RAW264.7 macrophages, and increased cholesterol efflux from these cells.421
Finally, it should be highlighted that mifepristone has frequently been used in a mifepristone-inducible transfection system to stabilize and increase ABCA1 expression in ABCA1-transfected baby hamster kidney (BHK)-21 cells. This ABCA1 induction could be functionally confirmed by increased ABCA1-mediated cholesterol and phospholipid efflux.245,273,422
Synthetic compounds, HTS hits, and synthetic approaches The purinergic P2Y7 receptor antagonists AZ-1, AZ-2, and AZ10606120 increased ABCA1 mRNA and ABCA1 protein levels and resulted in enhanced cholesterol efflux from human CCFSTTG1 astrocytoma cells.423 The polychlorinated biphenyl quinone 2,3,5-trichloro-6-phenyl-[1,4]-benzo-quinone (PCB29-pQ)424 and the fluorescigenic pyrazoline derivative 5 (FPD5)425 increased Abca1 mRNA424 and ABCA1 protein425 content in RAW264.7 macrophages and reduced cholesterol content in these cells.424,425 In vivo, FPD5 reduced aortic lipid and cholesterol content and atherosclerotic lesions in Apoe knock-out C57BL/6 mice.
Inducers of other ABCA transporters ABCA2 and ABCA3 As detailed above, ABCA2 and ABCA3 are believed to contribute to multidrug resistance in cancer.171,232-239,241,242 In human K562 leukemia cells, it was demonstrated that the tyrosine kinase inhibitor (TKI) imatinib induced increased levels of ABCA2 mRNA and ABCA2 protein.236 Furthermore, the TKIs dasatinib, imatinib, and nilotinib increased ABCA3 mRNA levels in various cancer cell lines as well as in TKI-treated leukemia patients.426 The antimetabolite 5-fluorouracil (5-FU) induced expression of ABCA3 mRNA in a cholangiocarcinoma cell line,427 and methotrexate increased ABCA2 and ABCA3 mRNA in a leukemia cell line.242 Finally, the steroid hormone progesterone,179 the antibiotic sulfoxaflor,420 and the endosomal cholesterol transport inhibitor U18666A179 induced ABCA2/ Abca2 transcripts420 in Aphis gossypii420 as well as in ABCA2-transfected Chinese hamster ovary (CHO) cells and HepG2 cells179
ABCA5 and ABCA6 As discussed earlier, cholesterol and its derivatives have been shown to induce ABCA1/Abca1 mRNA and/or ABCA1 protein levels.122,205,249,252,259,262-264,268,277,278,305-315,319-328 Induction by cholesterol has also been demonstrated for Abca5 mRNA and ABCA5 protein levels in RAW264.7 macrophages.321 This effect relied on the induction of Lxra, Lxrb, and Pparg. Consequently, several LXR and PPAR agonists increased Abca5 expression, including bezafibrate (PPAR-α, PPAR-β, and PPAR-γ; Abca5 mRNA and ABCA5 protein), GW3965 (LXR; Abca5 mRNA), rosiglitazone (PPAR-γ; Abca5 mRNA), and troglitazone (PPAR-γ; Abca5 mRNA) in murine RAW264.7 macrophages.321 In addition, the HMG-CoA-reductase inhibitor atorvastatin increased Abca5 mRNA and ABCA5 protein levels.321 Interestingly, the ABCA1 inhibitor tacrolimus245 showed induction of ABCA5 mRNA in human brain microvascular endothelial cells.428
The HMG-CoA-reductase inhibitors lovastatin and mevastatin resulted in an induction of ABCA6 mRNA in the human endothelial cell line EA.hy926.429 Finally, in an Abca12 pig model of the rare and lethal skin disease Harlequin ichthyosis, it was demonstrated that treatment with the synthetic retinoid acitretin leads to a compensatory induction of Abca6 mRNA.430
ABCA7 Similarly to ABCA1,202 the sterane derivative ponasterone A increased both ABCA7 protein expression and ABCA7-mediated transport, mainly of phospholipids, but also of cholesterol to a small extent.202
HMG-CoA-reductase inhibitors were described above to interfere with ABCA1/ Abca1312,318,330,343,362,411 and Abca5321 expression. In addition, certain compounds were also demonstrated to interfere with Abca7 expression.205,431 These include pravastatin205,431 and rosuvastatin (Figure 2).431 These agents increased Abca7 mRNA and ABCA7 protein levels in vitro,205,431 whilst pravastatin had the same effects in vivo in murine peritoneal macrophages.431 Surprisingly, this increase of Abca7 mRNA and ABCA7 protein levels was accompanied by a downregulation of Lxra and upregulation of Srebp2 in vitro.431 Functionally, pravastatin and rosuvastatin reduced intracellular cholesterol content431 and induced phagocytosis in vitro and in vivo.431 These effects occurred in response to an ABCA1 downregulation by HMG-CoA-reductase inhibitors as described earlier.312,321,384,432,439,429 Due to their functional similarity, the upregulation of ABCA7 could be a compensatory mechanism to counteract the loss of ABCA1.198 Similarly, the observed Lxra down- and Srebp up-regulation may be a compensatory mechanism to counteract the loss of intracellular cholesterol.
Finally, as described for ABCA1,422 exposure of ABCA7-transfected BHK-21 cells to mifepristone increased ABCA7 protein content and ABCA7-mediated transport of phospholipids and, to a much lesser extent, of cholesterol.422
ABCA8 ABCA8 mRNA and ABCA8 protein content were induced by gemcitabine in PANC-1 and CFPAC-1 human pancreatic cancer cells.221 In rat liver, an induction of Abca8 was demonstrated via microarray analysis of cDNA when the rats were exposed to polyethyleneglycol-block-polylactide nanoparticles.433
ABCA12 Several LXR and PPAR agonists induced ABCA12/Abca12 expression, such as 22-(R)-hydroxycholesterol (LXR),434 TO901317 (LXR),430,434 ciglitazone (PPAR-γ),434 GI 251929X (PPAR-γ),434 troglitazone (PPAR-γ),434 ceramide N-hexanoyl-D-erythro-sphingosine (PPAR-δ),435 and GW610742 (PPAR-δ).434
Interestingly, inhibition of certain enzymes to prevent ceramide processing elevated intracellular ceramide content and subsequently ABCA12 mRNA levels.435 These enzymes include, for example, the glycosyl-ceramide-transferase synthase [D threo-1-phenyl-2-hexadecanoylamino-3-morpholino-1-pro-panol (D-PPMP), D-threo-1-phenyl-2-palmitoyl-3-pyrrolidinopropanol (D-PPPP / P4) and DL-threo-1-phenyl-2-de-canoylamino-3-morpholino-1-propanol (D-DDMP)], the sphingomyelin synthase [tricyclo[5.2.1.02,6]decanyl)ethanedithioic acid (D609 xanthate)], as well as the ceramidase [D-erythro-2-tetradecanoylamino-1-phenyl-1-propanol (D-MAPP) and (D NMAPPD / B13)].435
Downregulators ABCA1 LXR and RXR pathways – intrinsic substrates The intrinsic metabolite asymmetric dimethylarginine (ADMA) reduced Abca1 mRNA and ABCA1 protein levels in human and murine J744 macrophages in combination with oxLDL, resulting in increased intracellular cholesterol and triglyceride levels.392 This was accompanied by decreased efflux of cholesterol from these cells. The authors suggested a negative effect on the LXR-α pathway. In this regard, the LXR-α downregulator homocysteine significantly reduced ABCA1/Abca1 mRNA and ABCA1 protein expression in vitro in THP-1 macrophages as well as in vivo in macrophages from Apoe knock-out C57BL/6 mice.335 The cattle metabolite dipeptide phenylalanine-proline decreased ABCA1 mRNA and ABCA1 protein levels in human colorectal adenocarcinoma-derived CaCo-2 cells.436 The observed downregulation of LXRB mRNA could explain the negative impact on ABCA1 expression. In vivo, the jejunal Abca1 mRNA levels were decreased in Wistar rats.436
The ABCA1 substrate α-tocopherol230 reduced ABCA1/Abca1 mRNA levels in vitro and in vivo.231 The same effects were observed for γ-tocopherol in vitro, most likely through the same mechanism. The authors suggested a negative impact on the LXR pathway due to deprived oxycholesterol derivatives after α-tocopherol treatment both in vitro in Hep3B cells and in vivo in rat liver.231
LXR and RXR pathways - sterane and sterane-like natural compounds Cholesterol and its derivatives have extensively been used to induce ABCA1/Abca1 expression122,205,249,252,259,262-264,268,277,278,305-315,319-328 However, mid-term exposure to excess cholesterol decreased ABCA1 expression though a negative impact on Lxra, Lxrb, and Pparg expression.321 Similar observations have been made for the sterol derivative dexamethasone, which also reduced ABCA1/Abca1 mRNA and ABCA1 protein expression in vitro and in vivo by downregulation of LXRA/Lxra mRNA and LXR-α protein levels as well as upregulation of Srebp2 and HMG-CoA-reductase gene expression (Hmgcr).437 Finally, an Abca1 mRNA reduction was observed in murine RAW264.7 macrophages for the Thelenota ananas-derived saponin desulfated holothurin A.438 Interestingly, Hmgcr was downregulated after exposure to desulfated holothurin A, which contradicts other findings.437
LXR and RXR pathways – other natural compounds Certain chalcone derivatives also caused reduced expression of ABCA1 protein.363 In addition, lipopolysaccharides reduced ABCA1 protein content in endometrial endothelial cells from C57BL/6 mice, which was accompanied by increased cholesterol levels in these cells.374 A parallel reduction in LXR-α protein was also observed. Finally, the carcinogenic agent N-nitrosodiethylamine (NDEA) demonstrated in vivo in Wistar albino rats a downregulation of Lxra and Lxrb mRNA as well as LXR-α and LXR-β protein levels and, subsequently, ABCA1 protein.368
LXR and RXR pathways – synthetic compounds and HTS hits In terms of other LXR antagonists and downregulators, GSK2033 (Figure 2),272,330,333 5CPPSS-50,357 and SR9243333 reduced ABCA1 mRNA and ABCA1 protein expression.272,330,333,357
HMG-CoA-reductase pathways – intrinsic substrates and pharmacological drugs The peptide hormone angiotensin II reduced cholesterol efflux from murine peritoneal macrophages.439 This reduction could be reversed by the angiotensin II receptor antagonist losartan. The authors concluded that ABCA1 was not involved in this process, as no concurrent change in Abca1 expression was observed.439 However, in another report, angiotensin II indeed demonstrated a reduction of ABCA1 mRNA and ABCA1 protein levels in human podocytes.440 The authors concluded a contribution of the HMG-CoA-reductase, SREBP1, and SREBP2.440
Geranylgeraniol pyrophosphate (GGPP; Figure 2), a product of the mevalonate pathway, reduced ABCA1 mRNA expression in human macrophages, which was blocked by the prenylation inhibitors L836,978 and L-839,867.314 In addition, a reduction of ABCA1-mediated cholesterol export was observed, which is also true for mevalonate itself.318 GGPP was used as a standard ABCA1 downregulator in certain studies.279,354,366
As discussed above, atorvastatin,343 fluvastatin,312 pitavastatin,318,343 and simvasta-tin312,343 have been shown to increase ABCA1/Abca1 mRNA levels,312,343 and to enhance ABCA1-mediated cholesterol efflux.318 However, atorvastatin,312,321,384 fluvastatin,312 pitavastatin432 and simvastatin312,384 have also been reported to reduce ABCA1/Abca1 transcription312,321,384,432 and ABCA1-mediated cholesterol efflux.384,431 These observations are in agreement with other reports on HMG-CoA-reductase inhibitors that downregulated ABCA1.312,431 In particular, lovastatin,312 mevastatin (compactin),412 pravastatin,431 and rosuvastatin431,441 reduced ABCA1/Abca1 mRNA312,431 and ABCA1 protein431 levels. These findings are expected given that the loss of cholesterol by interruption of cholesterol synthesis leads to a compensatory reduction of cholesterol efflux.314384,412 The contradictory results relating to ABCA1 may be caused by the use of variable experimental conditions between studies, such as different cell lines, assay methodologies, or small-molecule-related aspects, such as concentration, distribution, and protein binding.
Finally, a similar interconnection between HMG-CoA and ABCA1 was drawn for the antineoplastic agent mitotane, which downregulated ABCA1 mRNA441 and increased intracellular cholesterol levels.333,441 However, mitotane in combination with LXR antagonists and LXR downregulators had an inverse effect on mRNA regulation, increasing ABCA1 expression.327
PKC pathway - intrinsic substrates Interestingly, it was also demonstrated that long-term exposure to low concentrations of 8-Br-cAMP, a standard ABCA1/Abca1 inducer,230,249,255,266,290,292 led to decreased APOE secretion from human monocyte-derived macrophages.266 APOE secretion can be considered as a surrogate marker for ABCA1-mediated cholesterol transport.
PPAR pathway – pharmacological drugs and synthetic compounds Regarding the important PPAR pathway, it must be noted that troglitazone, indicated above as an ABCA1 inducer,268 was also reported to downregulate ABCA1 transcription.321 These inconsistent effects may be explained partially by the different concentrations used (1 μM vs 10 μM),268,321 but may also be related to cross-talk between the PPAR, LXR, and mevalonate pathways. The PPAR-γ antagonist GW9662 (Figure 2) reduced ABCA1 protein levels.406
Other ABCA1 downregulators – natural compounds Other small-molecules have been reported to act as ABCA1/Abca1 downregulators, acting independently of the previously mentioned LXR, RXR, PPAR, and HMG-CoA-reductase pathways. Natural compounds such as α,β-unsaturated carbonyl derivative acrolein,442 the polyphenol bisphenol A,443 and the polyphenol 1,2,3,4,6 penta-O-galloyl-β-D-glucose444 demonstrated an Abca1 mRNA443,444 and ABCA1 protein442 downregulation in vitro443,442 and in vivo.444 The effect of acrolein could be abrogated by 3-hydroxytyrosol,442 an inducer of ABCA1 protein content.445
SREBP2 has been demonstrated to be targeted by EGCG in high fat diet-fed transgenic SREBP+/+ Wistar rats, resulting in Abca1 mRNA downregulation, while an Abca1 mRNA upregulation could be observed under the same conditions in SREBP knock-out Wistar rats.446
Other ABCA1 downregulators – pharmacological drugs Exposure of the human non-small cell lung cancer lines A549 and H358 to the antiepileptic drug valproate led to downregulation of ABCA1 mRNA and ABCA1 protein levels through a histone deacetylase 2-(HDAC2)-mediated mechanism. In parallel, the authors observed an increased sensitivity of these cells to cisplatin.447
The selective estrogen receptor modulators raloxifene, tamoxifen, and toremifene were reported to reduce ABCA1 protein content in THP-1 macrophages along with decreased cholesterol efflux and increased intracellular cholesterol levels.341 Tamoxifen and raloxifene treatment decreased serum HDL-cholesterol levels in mice. In addition, tamoxifen reduced cholesterol levels in serum, liver, and feces of mice after injection with cholesterol-loaded macrophages.341 Interestingly, the downregulation of ABCA1 protein content by these estrogen receptor modulators could not be demonstrated for murine liver, indicating a macrophage-specific effect.341
Varenicline, a drug used in smoking cessation, was shown in vivo to promote aortic atherosclerotic lesions in Apoe knock-out C57BL/6 mice.417,448 The authors demonstrated that intracellular lipid content in peritoneal macrophages was increased, and a decreased ABCA1 protein expression was confirmed in vitro in RAW264.7 macrophages. Finally, the antineoplastic agent gefitinib reduced ABCA1 protein content in various non-small cell lung cancer cell lines.400
Other ABCA1 downregulators – synthetic compounds The plasticizer dibutyl phthalate389 and the PI3K/AKT inhibitor LY294002421 reduced ABCA1 mRNA389 and ABCA1 protein389,421 expression and increased cellular cholesterol and lipid levels389 in human389 and murine421 macrophages.
The sphingosine kinase 1 and 2 inhibitor 4-{[4-(4-chlorophenyl)-2-thiazolyl]amino}phenol was demonstrated to downregulate ABCA1 protein expression in murine primary macrophages, which was dependent on the sphingosine kinase 2 as well as the sphingosine-1-phosphate receptor.306 This ABCA1 protein downregulation was accompanied by a reduced cholesterol efflux.
The acyl coenzyme A cholesteryl acyl transferase (ACAT) inhibitor ATR-101 reduced ABCA1 mRNA levels and induced an increase in intracellular cholesterol content in H295R cells.251 The authors suggested that this was caused by inhibition of ABCA1 but provided no clear proof of direct inhibition of ABCA1. Therefore, this compound was classified as a downregulator.
Other ABCA transporters ABCA2 and ABCA3 Compared to ABCA1, knowledge relating to downregulators of the other ABCA transporters is very limited. As discussed above, human leukemia cells exposed to imatinib displayed increased ABCA2 mRNA and ABCA2 protein expression.236 Celecoxib abrogated this effect.236 A similar observation was reported for ABCA3, where the anti-inflammatory drug indomethacin and the ABCA1 inhibitor sirolimus245 (Figure 2) downregulated ABCA3 mRNA in various cancer cell lines.426,449,450 This treatment also resulted in a sensitization of these cell lines toward the TKIs dasatinib, imatinib, and nilotinib when treated with indomethacin.426
Other compounds were also reported to downregulate ABCA3/Abca3 including the flavonoid genistein,451 lipopolysaccharides452 – already demonstrated above as ABCA1 protein downregulators374 – and the translocator protein ligand PK11195.453 The effect of lipopolysaccharides could be abrogated by ascorbic acid (vitamin C).
ABCA5–ABCA9 Interestingly, the ABCA8 inhibitor222 and ABCA1 protein inducer253 digoxin downregulated Abca5 and Abca7–9 in murine liver.454 The HMG-CoA-reductase inhibitors lovastatin and mevastatin downregulated ABCA6 mRNA in human umbilical vein endothelial cells.429 The cholesterol derivative 25-hydroxycholesterol, which was introduced above as an ABCA1 mRNA inducer,327 showed the opposite effect on ABCA7 mRNA.324 This finding is in agreement with a report stating that excess cholesterol reduced ABCA7 protein content in both human and murine fibroblasts.205
Stabilizers of ABCA transporters Stabilizers are compounds that promote functional activity of ABC transporters through increasing their presence at the site of action (e.g., the cell membrane) either without interfering with mRNA or protein levels, or in addition to these effects. The categorization is difficult, as the necessary information regarding many modulators of ABCA transporters is lacking and the underlying mode of modulation cannot be precisely identified. In this section, we consider only those modulators which predominantly interfere with ABCA1 trafficking, with relatively minor or no additional modes of action/modulation. Stabilizers are of particular interest, as they may represent a novel generation of functional ABC transporter activators, expanding treatment options for several diseases, particularly AD.
ABCA1 Probucol and cyclosporine A were demonstrated above to decrease ABCA1 turnover and increasing ABCA1 protein content at the cell membrane.246,275 Arakawa et al. demonstrated that the probucol metabolites spiroquinone and diphenoquinone did not inhibit ABCA1-mediated transport like their parent compound but rather increased the fraction of functional ABCA1 in the cell membrane.275 This stabilization led to increased cholesterol and phospholipid efflux. Both effects were observed at very low nanomolar concentrations,275 while Abca1 mRNA remained stable.275 Strikingly, spiroquinone and diphenoquinone decreased vascular lipid deposits in vivo in cholesterol-fed rabbits,275 which may be of relevance for AD and potentially other neurodegenerative diseases.
A similar mode of stabilization, albeit with less potency and no in vivo confirmation, has been observed for the flavonoid wogonin,254 the olive oil-derived compound erythrodiol,395 and certain thiol proteinase inhibitors, in particular N-acetyl-Leu-Leu-norleucinal and leupeptin.316,386 Finally, the ABCA1 mRNA and ABCA1 protein inducer testosterone was demonstrated to promote ABCA1 trafficking to the cell membrane.357
Other ABCA transporters The cystic fibrosis transmembrane conductance regulator (CFTR; ABCC7) correctors C13,455 C14,455 C17,455 genistein,456 and ivacaftor (Figure 2)456 were demonstrated to rescue ABCA3 mutants by increasing total ABCA3 mutant protein levels,455 promoting subcellular targeting of ABCA3 into vesicular bodies,455 and improving lipid transport function of ABCA3.456 Furthermore, the correctors lumacaftor (VX-809; Figure 2), C3, and C4, and C18 increased the presence of ABCA4 at the cell membrane in ABCA4-overexpressing HEK293 cells, indicating promotion of ABCA4 trafficking to the plasma membrane.457,458 Promotion of trafficking has already been demonstrated for other ABC transporters, such as ABCC123,24 and ABCC7.459 Hence, this mechanism represents a new potential therapeutic option for ABCA transporter-related AD. As proposed for ABCC7,460 the authors suggested a direct binding of the correctors to the ABCA4 protein,457 which has not yet been proven.
In an Abca12 pig model of Harlequin ichthyosis, acitretin (Figure 2) treatment resulted in a redistribution of ABCA12 in the skin compared to wild-type pigs, and thus, a higher survival rate.430
Destabilizers of ABCA transporters Natural compounds In contrast to compounds that promote trafficking of functional ABCA1 to the plasma membrane, other compounds that have the opposite effect have been named ‘destablizers’. So far, only agents targeting ABCA1 are known. The lactone antibiotic brefeldin A (Figure 2) interfered with ABCA1 cell-surface localization, recycling, and intracellular trafficking.387,461-463 These effects were at least in part dependent on the interaction with brefeldin 1-inhibited guanine nucleotide exchange protein (BIG1).461 This interference reduced the functional fraction of ABCA1 and, consequently, ABCA1-mediated cholesterol and phospholipid transport.255 Similar observations have been made for the polyether-antibiotics monensin, which reduced ABCA1 turnover and trapped it inside endo- and lysosomes. Subsequently, monensin reduced the functional presence of ABCA1 at the cell surface,464 lowered cholesterol efflux,463 and increased intracellular cholesterol content.463,464 The same was demonstrated for nigericin, another polyether-antibiotic, which increased intracellular cholesterol concentration,463 and inhibited ABCA1-mediated cholesterol efflux from RAW264.7 macrophages.385 Inhibition of intracellular organelle transport as suggested for brefeldin A387,461-463 and monensin463,464 likely applies to nigericin as well.463,465 In addition, the endoplasmic reticulum stress promotor, tunicamycin, also reduced ABCA1 protein levels.360,466 This ‘downregulation’ is most likely mediated though stress-induced impaired ABCA1 trafficking and/or increased ABCA1 degradation.466 However, in terms of selective targeting of ABCA1 in particular, or ABCA transporters in general, these agents are less suitable as in vivo agents and serve better as in vitro controls.
The palmitic acid derivative 2-bromopalmitate (Figure 2) inhibited trafficking of ABCA1 to the plasma membrane and reduced ABCA1-mediated cholesterol efflux.273,467 However, the observed effect that ABCA1 did not translocate to the cell membrane in HEK293/ABCA1 cells467 has not been demonstrated in BHK-21/ABCA1 cells.273
Pharmacological drugs Interestingly, the experimental anticancer drug serdemetan (JNJ-26854165) was demonstrated to induce Abca1 mRNA levels but reduce ABCA1-mediated cholesterol efflux.468 The Abca1 mRNA induction was due to induction of Lxra and Lxrb. The Abca1 mRNA increase was also reflected at the protein level, which increased within 48 hours of exposure to serdemetan before a sudden decrease occurred. The authors also showed that ABCA1 turnover and degradation were increased. Thus, serdemetan can be considered a destabilizer.
Synthetic compounds Cycloheximide was frequently used to interrupt intracellular trafficking of vesicles, including ABCA1 containing endo- and lysosomes.387,464,468
As mentioned earlier, ABCA1 is stabilized by N-acetyl-Leu-Leu-norleucinal.316,386 This stabilization could be abrogated by the protein kinase C inhibitor Gö6976, which affected not only ABCA1 protein content, but also cholesterol and phospholipid transport.386
PART II: PIPELINE DEVELOPMENT TO GAIN NOVEL DIAGNOSTICS AND THERAPEUTICS
In silico methodologies to predict novel lead structures
Rational drug design is the innovative process of identifying pharmaceutically relevant drug candidates. It is based on the information obtained in association with the drug target, e.g., ABC transporters. In the following section, we will discuss computational approaches for in silico operations that help to identify novel lead molecules for potential diagnostic and therapeutic application.
Structure-based drug design
The development of computational methodologies for structure-based drug design to understand the relationship between transporter sequence/structure and function depends on the availability of structural as well as biological information. Recent advances in experimental approaches for structure determination have facilitated high-quality depictions of the structures of a growing number of ABC transporters in different conformational states.469 These experimental approaches include in particular X-ray crystallography and cryo-electron microscopy (cryo-EM).
Recently, the cryo-EM structures of human ABCA1470 and human ABCA4471-473 with resolutions of 4.1 Å and 3.3–3.6 Å, respectively, were reported. In addition, a cryo-EM structure of human ABCA7 has been announced474 on bioRxiv (biorxiv.org), which was, however, not published to this date (PDB ID: 7KQC). Nevertheless, a homology model of ABCA7 has been recently developed.475 Figure 4 shows the structures of ABCA1, ABCA4, and ABCA7 as determined by cryo-EM as well as homology modelling.
Figure 4. Available structures of ABCA transporters: the cryo-EM structures of human ABCA1470 (very left; PDB ID 5XJY) and ABCA4 [left (PDB ID 7LKP, middle (PDB ID 7E7I), and right (PDB ID 7M1Q)]471-473 as well as the homology model developed for human ABCA7 (very right).475 All three transporters are typical ABCA transporters with three crucial structural parts: two nucleotide-binding domains (NBDs; intracellular), two membrane-spanning domains [MSDs (2 x 6 transmembrane helices TMs); inter-membrane space], and two large extracellular domains (ECDs; extracellular).
Considering the available structural knowledge, a ‘common’ ABCA transporter possesses a very long amino acid sequence (>2000 amino acids) and consists of two membrane-spanning domains (MSD1 and MSD2) each composed of six transmembrane helices (TM1–6 and TM7–12). These MSDs are followed by a cytoplasmic region comprising a nucleotide-binding domain (NBD1 and NBD2) and a small regulatory (R1 and R2) domain, which have been proposed to stabilize the interaction between NBD1 and NBD2470,473 and were found to strongly interact with each another in the absence of ATP.471,472
ABCA transporters are ‘type II transporters’ in which the MSDs indeed form a tunnel for substrate translocation from the cytosol to the lumen, however, represent separate entities without swapping/twisting of the MSDs, as this is the case with classical ‘type I transporters’ like ABCB1.476 Most TMs are completely exposed to the hydrophobic environment of the membrane, which could promote the attraction and binding of fat-soluble cholesterol as well as phospholipids before guidance to and through the substrate translocation tunnel, and which hosts several cholesterol and phospholipid binding sites.470-474
A unique feature amongst ABCA transporters in comparison to other ABC transporters is the existence of two large extracellular domains (ECD1 and ECD2). These domains together form a channel embedded in hydrophobic amino acids470-472 and are believed to facilitate intermediate storage of cholesterol470 and phospholipids. They have also been suggested as the primary binding site of APOA1,471,477 as indicated by the latest data on ABCA4.471 A large gap exists between the ECDs and MSDs, pointing to strong conformational changes that are required for ABCA transporter function.470 Another common feature amongst ABCA transporters are four intracellular and extracellular helices (IH1–4 and EH1–4), which are believed to provide the necessary flexibility for interaction between the MSDs and NBDs in the substrate translocation process,478 and were suggested to enable proper folding and function of these transporters.471
Of important note is that ABCA1 and ABCA4 share sequential and structural similarities with the ABCG family, in particular with ABCG5/ABCG8,470 which is the model type II transporter.478 This similarity suggests an evolutionary relevance amongst various ABC transporter subfamilies. More importantly, conserved sequential and structural similarities also support the translation of knowledge gained on other ABC transporter subfamilies to ABCA transporters.470,472 This is of particular interest when novel lead structures for new pharmacological targets, in this case under-studied ABC transporters,18 are focused,6,18 and specific binding sites located within the MSDs or NBDs are targeted.
Based on the sequence information of ABC transporters within the same family, homology-modeling techniques are the preferred choice for structure determination and binding site elucidation if these subtypes do not yield X-ray or cryo-EM structures. This methodology is of particular relevance for closely related homologs with high medical relevance,198 such as ABCA7 (similarity A1/A7: 54%; similarity A4/A7: 49%).200 The generated homology models can be refined further by molecular dynamics simulation, in which the transporter movement (‘trajectory’) is simulated to potentially unravel relevant transporter conformations. Very recently, potential ABCA1 drug binding sites have been proposed by this methodology,479 and an ABCA7 homology model has been developed for molecular docking experiments.475
Molecular docking is a very popular method for predicting binding orientations or poses of small-molecules within the transporter. Most often, the docking programs account for full conformational flexibility of ligands within the binding site, treating the protein as a rigid body. Binding site identification is an important prerequisite in the structure-based drug design implementation. In terms of ABC transporters, the search for binding hot spots and cavities on the entire volume of the protein (e.g., through blind docking) is necessary due to the general lack of information on binding sites of ABC transporters.
Recently, in search of highly effective modulators addressing ABCG2-mediated MDR, derivatives of quinazolines were synthesized and biologically assessed using a Hoechst 33342 accumulation assay.480 By utilizing the cryo-EM structure of ABCG2,481 molecular docking studies were performed using a fragment-based approach.482 This approach was used to gain insights into the molecular determinants involved in the formation of the transporter-substrate complex.480 Based on the docking studies, the putative binding site of the ABCG2 substrate, Hoechst 33342, and its interaction with the amino acids in the binding pocket was proposed.480 The predicted binding pose was rationalized based on the mutagenesis data reported in the literature483-487 and further confirmed with kinetic studies to determine the mode of inhibition.480 This subsequent structure-based approach led to the discovery of highly potent pyrimidine-based ABCG2 inhibitors,488,489 specifically by identifying a novel binding pocket of this transporter.488 In terms of ABCA transporters, molecular docking experiments with the newly derived ABCA7 homology model applying a set of diverse pan-ABC transporter inhibitors revealed a putative common ‘multitarget binding site’ identified within the transmembrane domains of ABCA7. It must be noted that the nucleotide binding domains are the most highly conserved regions amongst all ABC transporters, and hence, may also represent a(nother) multitarget binding site for certain drugs. However, the vast majority of data reported in the past hint to the transmembrane domains as the actual venue of bioactivity in terms of ABC transporter modulation.472
These results as described above475,480,488,489 give this methodology a high relevance in the drug development process in terms of novel lead molecules in general, and provide the basis for rationally designed structure-guided approaches for the identification of modulators of ABCA transporters in particular, as recently demonstrated for ABCA7.475
Ligand-based drug design
Similarity search The analysis of structure-activity relationships using ligand-based approaches is an essential component of medicinal chemistry and pharmacology of ABC transporters. This becomes evident as X-ray or cryo-EM structures of most ABC transporter subtypes are lacking to serve as suitable templates with sufficient similarity for generating homology models. Ligand-based approaches establish a correlation between the molecular structure of a small-molecule and the triggered biological response of the target. The chemical representation of the molecules is often expressed using descriptors, which are attributes that conserve the physicochemical information of the molecule. These descriptors refer to generic properties such as LogP, molecular weight, polar surface area, rotatable bonds, or molar refractivity. Alternatively, structural representations of the molecules can form fingerprints that portray existent molecular features of the molecule in a binary code. These fingerprints are, for example, path-like,490 or circular-based,491,492 such as MACCS or ECFP4, respectively. Utilizing these representations of molecules, similarity-driven virtual screenings can be applied. Here, molecules are extracted from a virtual library of millions or billions of compounds compared to the bioactive template molecule(s) according to the similarity principle. The abstract representation of molecules enables clustering of compounds, which is a methodology to categorize a diverse set of molecules. Moreover, these abstract representations can be used in different machine learning (artificial intelligence) approaches.
Pharmacophore modelling Another common approach is pharmacophore modelling, which analyzes a number of ligands with a common mechanism of action. The model is the ensemble of common chemical features that are required to ensure the molecular interaction of the ligands with the target, such as hydrogen bond donors and acceptors as well as aromatic and hydrophobic centers. The pharmacophore models are generated by extracting common molecular features through flexible alignment of the active biomolecules.493,494 This can be achieved by generating all possible conformations of the ligand and aligning them to determine the essential chemical features and molecular orientation to construct the pharmacophore model. The conformational flexibility of the ligands representing the chemical features is the key factor in the pharmacophore model generation.
Pattern analysis In addition to these classical computational approaches, similarity search and pharmacophore modelling, a pattern analysis approach (‘C@PA’ = computer-aided pattern analysis’) has been reported recently.18,19,495 Pattern analysis extracts both basic scaffolds and the statistical distribution of substructural elements amongst the template ligands. It works similarly to non-physicochemical properties-related fingerprints and conserves substructural features as they are present in the molecules. Pattern analysis has specifically been derived for the development of novel potent multitarget ABC transporter inhibitors. The basic operations were the categorization of bioactive molecules according to their inhibitory power against specific ABC transporters and their classification according to their selectivity profile. The respective classes can statistically be analyzed for both their basic scaffolds and/or their substructural composition to extract the desired pharmacological profile and target preferences. The generated model focused multitargeting of ABC transporters, and resulted in a biological hit rate of 21.7%.19 Adaption of the model (‘C@PA_1.2’) through additional non-statistical and exploratory measures increased the biological hit rate to 40%,18 and an additional extension of the model enabled the discovery of the ‘outer multitarget modulator landscape’, which represented weak multitarget bioactivities (>10 μM) supporting the discovery of a larger number of multitarget agents.495 The hit rates are impressive considering that this approach takes several targets with individual ‘ligand preferences’ into account. Furthermore, as several ABC transporters of distinct subfamilies were considered (ABCB1, ABCC1, ABCG2), the resultant multitarget agents open up the possibility to explore under-studied ABC transporters,18 in particular ABCA transporters in terms of AD.6,14
Combined approaches Apart from the individual use of these methodologies, combined approaches may lead to improved hit rates and better prediction capabilities with respect to bioactivity of small-molecules. This has in particular been demonstrated for a combined virtual screening approach using similarity search and pharmacophore modelling for the discovery of novel ABCC1 inhibitors.493 Also, certain pattern analysis approaches have used a data set derived from a similarity search and pharmacophore modelling approach, and hence, can also be considered a combined computational approach.18,495
In vitro methodologies to assess novel lead structures
The previous sections have already outlined the diverse testing systems that have been used to assess the modulatory effects of effectors toward ABCA transporters. The following section will highlight the ABCA transporter-expressing host systems and the related assays that can be implemented into the pipeline for the assessment of novel lead molecules as potential ABCA transporter diagnostics or therapeutics.
Host system of ABCA transporters
The transporter host system (ABCA transporter carrying unit) can be categorized into (i) living-cell-based or (ii) membrane preparation-/vesicle-based (including isolated and reconstituted proteins). The vast majority of biological investigations used living cells. Here, two different living cell-based transporter host systems can be differentiated: (i) native/induced/selected cells and (ii) transfected cells.
Native ABCA transporters-expressing living cells Native/induced/selected cells naturally express the respective ABCA transporter or have been exposed to a ‘standard’ inducer, for example, the ABCA1 inducers 22-(R)-hydroxycholesterol,122,205,249,252,259,262-264,268,277,278,305-315 TO901317,205,245,250,252,259,260,262,264,271,272,279,
280,282,308,310,317,319,322,324,326,328-345 or 8-Br cAMP,230,249,255,266,290,292 and overexpress the respective transporter in response (e.g., ABCA1). Most commonly, human or murine cells have been used. Table 4 summarizes the cell lines used to assess the ABCA transporter modulators discussed in the previous sections. It must be noted that the addressed pathways regulate also the overexpression of other ABC transporters. In terms of the studies of ABCA1, the co-expression (i.e., co-upregulation and co-downregulation) of other members, such as ABCG1, has frequently been observed.160,320,335,364,366,402,410,418,421,448
| Cell type | Cell line name | Origin | References |
|
| ABCA1 | | | |
| colorectal adenocarcinoma cells | CaCo-2 | human | 262,264,308,314,342,436 |
| lung adenocarcinoma cells | HCC827-GR
PC9-G2 | human | 337
337 |
| renal adenocarcinoma cells | 786-O
A498
ACHN
HK-2
SN12C
OS-RC-2 | human
human
human
human
human
human | 334
330
334,349
330
330
330 |
| adipocytes | 3T3 L-1 | mouse | 255 |
| adrenocortical carcinoma cells | H295R
MUC-1 | human
human | 333,441
333 |
| astrocytes | | human
mouse
rat | 279
229,279
281 |
| astrocytoma | CCFSTTG1 | human | 423 |
| peripheral blood mononuclear cells | PBMC | human | 411 |
| breast cancer cells | MCF-7 | human | 331 |
| pancreatic β-cells | INS-1 | mouse | 409 |
| cardiomyocytes | H9c2
HL-1 | rat
mouse | 253
250 |
| aortic endothelial cells | HAEC | human | 263,269 |
| endometrial endothelial cells | | mouse | mouse |
| umbilical vein endothelial cells | HUVEC | human | 269,364,442,496 |
| epithelial cells | BEAS-B2 | human | 322 |
| lung epithelial cells | | mouse | 311 |
| pigment epithelial cells | | human | 257 |
| mouse mammalian epithelial cells | MMEC | mouse | 350 |
| aortic smooth muscle cells | SMC | human | 269 |
| vascular smooth muscle cells | VSMC | unspecified origin | 332 |
| fibroblasts | primary hip skin
WI-38 (embryonic)
WI38VA13 (embryonic)
BALB/3T3
Swiss 3T3 | human
human
human
mouse
mouse | 230,260
205,246
277
275
312 |
| granulosa cells | | rat | 443 |
| hair follicles | | human | 282 |
| hepatoma | Fu5AH
Hep3B
HepG2
McARH7777 | rat
rat
human
rat
rat | 318
231
309,342,348,379
280,312,317,367,381
343 |
| insulinoma cells | INS-1 | rat | 405 |
| keratinocytes | | human | 282 |
| embryonic kidney cells | | human | 312 |
non-small cell lung cancer cells
| A549
H1650
H1975
H358
PC-9/GR | human
human
human
human
human | 322,447
400
400
447
400 |
| liver cells | L02 | human | 406 |
| mantle cell lymphoma | MCL | human | 468 |
| macrophages | primary | human | 268,305,339,396,398 |
| | | mouse | 306,312,313,320,329,341,360,366,439,448 |
| | HD11 | chicken | 356 |
| | J774.A1 | mouse | 252,254,255,259,265,271,278,289-292,384,392,393 |
| | RAW264.7 | mouse | 249,312,313,321,336,339,342,352,360,365,367,369,375,
376,381,385,399,402,404,406,408,410,416-419,421,424,
425,438,442,448,497 |
| | THP-1 | human | 231,245,249,256,268,272,275,292,308,310,312-316,321,
328,335,338,339,341,342,360,363,364,366,377,384,
388-397 |
| | U937 | human | 307 |
| microglia | primary
BV2
retinal (Müller cells) | rat
mouse
mouse | 355
126,353,380
323 |
| multiple myeloma | MM | human | 468 |
| neuroblastoma | Neuro-2a | murine | 359 |
| neutrophils | primary | human | 339 |
| nephron cells | A6 | frog | 258 |
| periodontal ligament stem cells | | human | 325 |
| pheochromocytoma | PC12 | rat | 280 |
| podocytes | | human | 440 |
| retina cells | ARPE-19 | human | 354 |
| oral squamous cell carcinoma cells | CAL27 | human | 371 |
| trophoblasts | BeWo | human | 437 |
|
|
|
| ABCA2 | | | |
| hepatoma | HepG2 | rat | 179 |
| ovary carcinoma | SKEM | human | 238 |
|
|
|
| ABCA3 | | | |
| cholangiocarcinoma | M214-5FUR | human | 427 |
| lung epithelial cells | MLE-12 | mouse | 452 |
| hepatoma | HepG2 | rat | 451 |
| leukemia | primary (acute myeloid)
BV173
K562
LAMA83 | human
human
human
human | 234
234,236
234
235 |
| lung cancer | A549
NCI-H1650
NCI-H1975 | human
human
human | 241
241
241 |
|
|
|
| ABCA5 | | | |
| brain microvascular endothelial cells | HBMEC | human | 428 |
macrophages
| RAW264.7
THP | mouse
human | 321
321 |
|
|
|
| ABCA7 | | | |
| fibroblasts | BALB/3T3
WI-38 | mouse
human | 205
205 |
| macrophages | J774.A1 | mouse | 431 |
|
|
|
In terms of ABCA1, most studies have been conducted with human THP1,231,245,249,256,268,272,275,292,308,310,312-316,321,328,335,338,339,341,342,360,363,364,366,377,384,388-397 murine J774.A1,252,254,255,259,265,271,278,289-292,384,392,393 or murine RAW264.7 macrophages.
230,249,312,313,321,336,339,
342,352,360,365,367,369,375,376,381,385,399,402,404,406,408,410,416-419,421,424,425,438,442,448 In the set-up of a drug development pipeline, these cell lines are the backbone of the in vitro assessment of potential candidates.
Regarding other ABCA transporters, the situation is much more complicated due to the lack of cell lines that naturally (and almost exclusively) express the respective ABCA transporter. Consequently, these ABCA transporters are much less studied and well-established. However, transfected cell lines are of great help to study one particular transporter instead of using native cell lines that may co-express several members.
ABCA transporters-transfected living cells In terms of ABCA1, cell lines transfected with human ABCA1 have often been used, e.g., human embryonic kidney (HEK) cells (HEK293/ABCA1)171,201,202,249,260,267,270,275,329,352,386,464,467,498,499 and baby hamster kidney (BHK) cells (BHK-21/ABCA1).230,245,273,292,422 These transporter host systems have also been used to study other transporters, ABCA2,498,500 ABCA3,235,241,498 ABCA4,133-136,201,457,458,501,502 ABCA5,503 ABCA7,201,202,386,422,498 ABCA8,10 ABCA12,498 and ABCA13.48
Transfected cells often express lower levels of the introduced transporter than native cell lines, which is a problem if the host cell lines (e.g., HEK or BHK-21) naturally express other ABC transporters as well. However, these transporter host systems are suitable to confirm results, and might be the only possibility to address ABCA transporters other than ABCA1.
Isolated ABCA transport proteins
Finally, apart from intact cells, vesicles of enriched or purified/reconstituted ABCA transporters have also been used to assess transporter function. Compared with living-cell based assays, this kind of host system is rarely represented in the literature regarding ABCA transporters.133-139,201,499-502,504-506 Specifically ATPase assays are popular to assess functional ABC transporter modulation.23,24,507-510 While transport protein purification and reconstitution in vesicles or nano discs requires advanced engineering, and is expensive and resource-consuming, membrane preparations of transporters, in particular for ATPase assays, are much more feasible. However, this method has been used somewhat scarcely for ABCA transporter function assessment.133-135,137-139,201,499-502,504-506
Functional assessment of ABCA transporters
Two groups of tracers have been established in terms of ABCA transporter function: (i) radiolabeled substrates,250,272,305,306,338,339,354,364,366,393,395,404,419,511,512,136,222,230,245,249,253,255,259,260,262,264,265,267-270,
273,276,278,289-292,311,313,315,318,329,341,367,377,381,384,385,464,467,499,513 and (ii) fluorescent substrates.171,201,238,
251,252,254,256,258,261,271,282,308,319,321,330,332,335,342,360,379,389,390,392,397,402,406,455,456,468,514-518
Radiolabeled tracers of ABCA transport function In terms of radiolabeled substrates, cholesterol is by far the most frequently used genuineABCA1 substrate,230,245,249,255,260,264,265,267-270,272,273,276,278,289-292,305,306,313,315,318,329,338,339,354,366,367,381,384,385,
393-395,404,408,419,464,467,499,512 followed by phospholipid(-components).249,255,267,269,273,311,464,467,514 However, other substrates have also been used. These substrates include mostly molecules with sterane scaffold, such as β-sitosterol (ABCA1)262 and estradiol-β-glucuronide (ABCA8).222 Moreover, lipid-like substrates have attracted attention, like sphingosine-1-phosphate (ABCA1),229,496 α-tocopherol (ABCA1),230 and ATRA (ABCA4).136 Notably, radiolabeled substrates are very effective in terms of accurate tracing of protein function, as these molecules are not changed in their molecular integrity in contrast to fluorescence probes.171,201,238,251,252,254,256,258,261,271,282,308,319,321,330,332,335,342,360,379,389,390,392,397,402,406,455,456,468,
514-518 On the downside, conducting these experiments is constrained to regulatory requirements and requires extensive staff training as well as expensive safety measures and laboratory equipment.
Fluorescent tracers of ABCA transport function Regarding fluorescent derivatives of cholesterol and phospholipids, two major types can be differentiated: (i) 7-nitro-2,1,3-benzooxadiazole (NBD) derivatives201,251,252,254,256,258,261,308,335,342,360,379,389,390,392,394,397,402,
406,408,468 and (ii) 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) derivatives.271,282,319,321,330,332,455,456,
515-517 Other fluorophore-labeled dyes have been reported, too, including the sterane analog dansyl-estramustine,171,238,518 and propargyl choline, which is processed in vitro into propargylated phospholipids.514
In addition to the stated fluorescent tracers of ABCA transport function, several other derivatives of other substrates can be proposed. For example, N-3-oxododecanoyl-L-homoserine lactone (3OC12-HSL) was suggested as ABCA1 substrate, but final proof was missing.519 Thus, it may be a suitable candidate for validation in a new set-up in vitro assay for ABCA1 (and potentially other ABCA transporters). Other examples of potential probes are fluorescenct dyes that stand in association with cellular cholesterol and phospholipid distribution and ABCA1-mediated cholesterol and phospholipid transport.516 These include, for example, β BODIPY FL C5-HPC, β-BODIPY FL C12-HPC, BODIPY TR ceramide, and Red/Green BODIPY PC-A2, amongst many others.520-522
Fluorescenct dyes are well-established tracers of ABC transporter function,18,19,23,24,284,480,488,489,493,507,
523-525 and the knowledge that has accumulated regarding the well-studied ABC transporters ABCB1, ABCC1, and ABCG2 can be transferred to ABCA transporters as well. However, the added fluorophore changes the molecular composition of the tracing molecules. This alteration inheres the potential risk of changing affinities and even the binding site(s) of these molecules, undermining functional-kinetic analyses regarding binding site determination and elucidation of the mode of action. Nevertheless, fluorescence probes are – if used and established correctly – extremely reliable, and can be used without regulatory restrictions and necessity of special equipment, except for microplate readers and/or flow cytometers.
Colorimetric determination of ABCA transport function – ATPase assays As mentioned above, ATPase assays have also been used to functionally analyze ABCA transporter function, in particular for ABCA1,201,499,505,506 ABCA2,500 ABCA3139,504 ABCA4,133-135,137,138,201,501,502 and ABCA7,201 although this methodology has been used somewhat rarely compared to other functional approaches. ATPase assays are based on the principle that the active transport of any substrate of ABC transporters consumes energy. This energy is derived from the cleavage of ATP to ADP and Pi, and can be detected by different methodologies.23,24,507-510,526 Table 5 highlights known ATPase modulators of ABCA transporters and the associated literature reports.
| Transporter | Modulator |
Mode of modulation | References |
| ABCA1 | ceramide (30 mol–%)
cholesterol (30 mol–%)
phosphatidylcholine (30 mol–%)
phosphatidylethanolamine (30 mol–%)
phosphatidylinositol (30 mol–%)
phosphatidylserine (30 mol–%)
sphingomyelin (30 mol–%) | inhibition
inhibition
activation
inhibition
inhibition
activation
activation | 201
201,499
201
201
201
201
201 |
| ABCA2 | methyl-β-cyclodextrin (u.c.a) | activation | 500 |
| ABCA4 | amiodarone (20–75 µM)
2-tert-butylanthraquinone (20–50 µM)
ceramide (30 mol–%)
cholesterol (30 mol–%)
dehydroabietylacetate (10–50 µM)
digitonin (10–180 µM)
N-ethylmaleimide (NEM; 1000 µM)
reduced glutathione (GSH; 1000 µM)
β-ionone (50–100 µM)
phosphatidylethanolamine (30 mol–%)
phosphatidylglycerol (30 mol–%)
phosphtidylinositol (30 mol–%)
11-cis-retinal (5–100 µM)
13-cis-retinal (5–100 µM)
ATRA (5–100 µM; EC50 = 10 µM)
all-trans-retinoic acid (20–100 µM)
all-trans-retinol (20–100 µM)
N-retinylidenephosphatidylethanolamine (40 µM) | activation
activation
inhibition
inhibition
activation
activation
inhibition
activation
activation
activation
activation
inhibition
activation
activation
activation
activation
activation
activation | 138
138
201
201
138
138
137
137
138
201
201
201
137,138
138
133-135,137,138
138
133,138
133 |
| ABCA7 | ceramide (30 mol–%)
cholesterol (30 mol–%)
phosphatidylcholine (30 mol–%)
phosphatidylethanolamine (30 mol–%)
phosphatidylserine (30 mol–%) | inhibition
inhibition
activation
activation
activation | 201
201
201
201
201 |
a u.c. = unspecified concentration
ATPase assays have been and still are popular in terms of functional ABC transporter modulation in general.23,24,507-510 Strikingly, the NBDs of ABC transporters are – in contrast to the various binding sites identified within the transmembrane domains of ABC transporters475 – highly conserved. This conservation enables targeting of ABCA NBDs by known ATPase modulators of other ABC transporters. Therefore, ABCA transporter function can be detected by methodologies that have already been established for other ABC transporters.23,24,507-510,526 This transfer of knowledge will be of great use to confirm obtained results from other functional ABCA transporter analyses.
Colorimetric determination of ABCA transport function – other detection methodologies As a final note, it must be mentioned that other colorimetric analyses were also used to quantify the ABCA transporter-mediated function, specifically for transport of cholesterol or choline-containing lipids, using commercially available assay kits.202,205,246,251,268,272,275,329-332,334,336,354,365,366,369,372,374-376,386-389,392,405,
406,416,418,421,422,424,425,441,511 However, these methodologies require time-consuming extraction processes of the lipids, and hence, are less suitable to track the function of ABCA transporters in real-time and to determine kinetic aspects of their cholesterol and lipid transport.
In rare instances, the extraction of lipid components was accomplished after incubation with a radioactive marker.246 While this is a valid methodology to accurately determine lipid components within cells, it increases workload and attracts regulatory constraints.
Gas-liquid chromatography has also been used in some reports.353,355 An extraction-free staining of cholesterol inside of cells was also demonstrated (filipin III251,331,333,341,358 or Oil Red O staining 330,332,364,366,369,375,388,389,392,393,399,402,410,417,421,425,448).
However, these systems are not suitable to track single-cell ABCA-mediated cholesterol or phospholipid transport.
Quantification of ABCA transporter regulation Besides qPCR and western blotting, ABCA transporter expression was reported in several studies using fluorimetric assays. This was accomplished with either (i) green fluorescent protein-(GFP)- tagged/labelled ABCA transporters235,241,261,275,386,422,464,504 or (ii) luciferase promotor-(LUC)-transfected271,309,319,352,367,379,381,405,
406,419,436,447 ABCA transporter cells in luciferase reporter gene assays.
In vivo assessment of clinical candidates
In vivo models play a key role in drug discovery. Although in vitro and cellular models are less expensive and less time consuming, in vivo models are needed to test ABCA modulators under physiological conditions. Safety, toxicity, and efficacy of a drug candidate must be tested in an in vivo model as a last step before transferring it to clinical evaluation. However, these models also have disadvantages. Animal studies are time consuming and require advanced personnel training and resources for maintaining the animals. In addition, although they are closer to humans than in vitro models, there are considerable physiological differences between species with respect to drug absorption, metabolism, and excretion, which may impede translatability. Furthermore, the use of animals in research has its ethical concerns. Thus, in recent years, research has been directed to reduce animal use and increase animal welfare.
In vivo models have previously been used to study the role of ABCA transporters in physiology and disease as described above. Thus, there are already available animal models for testing of ABCA modulators for the most prominent subtypes (Table 6). As stated above, these models represent the last step before clinical evaluation of potential small-molecule therapeutics in humans. Thus, after in silico identification and in vitro assessment, these in vivo models are the third column in the development of novel ABCA transporter diagnostics and therapeutics. In the following section, different in vivo models will be described in more detail.
| Transporter | Type | Species | Phenotype | References |
| ABCA1 | knock-out | mouse
| reduced cholesterol and plasma phospholipid levels
decreased brain APOE levels
poorly lipidated APOE
| 161-163
https://www.jax.org/strain/003897 |
| | overexpression | mouse | increased lipidation of APOE | 127 |
| ABCA2 | knock-out | mouse | reduced body weight, limb tremor, reduced sphingomyelin | https://www.jax.org/strain/033139
54,527 |
| ABCA3 | knock-out | mouse
| Knocked-out pups die within 1h after birth | 186,528,529 |
| | missense mutation | mouse | early macrophage predominant alveolitis which peaked at 8 weeks of age
| 530 |
| ABCA4 | knock-out | mouse | abnormal phospholipid composition, delayed dark adaptation | 531,532 |
| ABCA5 | knock-out | mouse | exophthalmos and collapsed thyroid gland, early death due to cardiac insufficiency | 123,131 |
| ABCA7 | knock-out
humanized | mouse
mouse | reduced microglia response
altered phagocytosis
increased β-secretase
under characterization, increase Aβ load | 124
Abca7tm1.1(ABCA7)Pahnk
MGI:6258226 |
| ABCA8 | knock-out | mouse | reduced plasma HDL
| 533 |
| | adenoviral overexpression | mouse | increased plasma HDL and cholesterol
| 533 |
| ABCA12 | - | - | not described
| https://www.jax.org/strain/033630 |
| ABCA13 | knock-out | mouse
monkey | deficits of prepulse inhibition
impaired neuronal formation, neurotransmitter alterations | 48
534 |
Knock-out mouse models
A genetic knock-out mouse model is an animal model in which one or more genes of interest have been deactivated or removed by means of gene targeting. Knock-out animals allow for direct investigation of the effect of a specific gene in an organism, as the loss of gene activity often causes phenotypic changes uncovering the function and biological mechanism of the targeted gene.535 Knock-out mice have become one of the most useful scientific tools to analyze the human genome and its potential roles in many diseases.535 Thus, knock-out animals are currently essential experimental tools for the investigation of genetic disorders and the evaluation of novel drugs.536 Furthermore, the current knowledge on genome editing using the CRISPR/Cas9 system makes generation of knock-out lines considerably faster than with the use of embryonic stem cells. To no surprise, this method has quickly become the most powerful tool for generating genetic models.537
Knock-out animal models are designed with two variables in mind: (i) where and (ii) when is the gene of interest deactivated. The simplest and most common approach is a constitutive, ubiquitous knock-out, i.e., the product protein is absent permanently in all cells of an organism. To overcome limitations of this broad approach, more refined models have been developed. These conditional models use Cre-Lox recombination to target a gene either in specific cell populations, at specific time points, or a combination of both. Here, the target gene is modified by inserting two loxP sites. The flanked gene segment can then be excised by the Cre recombinase. Cre activity, i.e., gene knock-out, can be limited to certain cell populations by appropriate promotor choice and/or linked to a tamoxifen-responsive element to control the exact time point at which the knock-out is induced.
Until now, several Abca animal knock-out models have been described, which are summarized in Table 6. These models are mainly mouse lines, except for ABCA13 (monkey).534 These animal models have contributed fundamentally to identifying the role of ABCA transporters in physiological conditions as well as in disease pathogenesis. In addition, these models can be used for novel drug testing, as they provide information about target specificity. If a drug is 100% specific for a transporter, knock-out of this transporter should completely abolish the drug’s effects observed in naïve animals. However, gene knock-outs often have phenotypical effects per se that need to be taken into account when evaluating drug effects.
RNAi models
The use of RNA interference (RNAi) is an alternative to knock-out models. This technique is based on post-transcriptional silencing of the targeted gene using siRNA molecules that are designed to bind to the target mRNA.538 This process will deactivate the mRNA using the cell’s own defense mechanism against pathogens. In contrast to standard knock-out models, this silencing is temporary as the siRNA molecule will be degraded but the gene transcription continues.527
To avoid this temporal limitation, short-hairpin RNA (shRNA) has been developed. This method is based on the use of vectors that incorporate into the cell DNA and encode for shRNA. After transcription, these vectors are processed into siRNA. These shRNAs are continuously transcribed, increasing reproducibility of results.539
Overexpression models
Similar to knock-out models, overexpression models can be used to investigate the function of a gene by evaluating the resultant phenotype. In addition, overexpression models have long been used for modeling diseases such as AD540 or PD.541
In the investigation of ABCA transporters, these models can resemble the effect of chronic activation of the transporters and may help to identify its physiological functions by evaluating the pathways upregulated in comparison to control animals.127
Humanized ABC transporter mouse models
Before it can be translated into clinical practice, each novel drug candidate must be tested in an in vivo model. However, the translational value of the animal model largely depends on whether the disease pathway under investigation is conserved between the two species. Therefore, replacing the original (e.g., murine) gene by the respective human gene likely improves the animal model, and thus, is beneficial for evaluating a novel drug’s efficacy and specificity in clinical practice.542 With this approach, mice can be used as tools for pre-clinical screening and efficacy evaluation of new drugs, given their improved ability to predict human responses to treatments.
Our group has previously established a humanized ABCC1 mouse model,543 and an ABCA7 model is under characterization. Here, we generated knock-in mouse models producing a chimeric protein that is completely human except for one amino acid.543 In addition, as this gene was flanked by loxP sites, this humanized model can be knocked out in specific cell populations and at a specific age.543 Models such as these represent the future of pre-clinical drug candidate evaluation.
In addition, Dallas et al. successfully generated a humanized ABCG2 mouse model.544 However, other models, such as humanized ABCB1 mice, were not successful despite multiple attempts.545
Disease models
In addition, all the models described above can also be used to study the role of a gene for the pathophysiology of specific diseases. For example, Abca knock-out models have been crossed with transgenic mice in order to study their potential role in AD.54,123,131,161-163,527 These studies have elucidated potential disease mechanisms involving ABCA transporters that cannot be studied in patients.
Moreover, once a drug is developed and its specificity is proven, disease models enable evaluation of the role of that specific transporter in the pathophysiology of the disease. At the same time, these results may be the first step to evaluate the potential of novel transporter modulators as therapy for the respective disease.
Imaging techniques
Lastly, in vivo imaging can be used for the development of new drugs. On the one hand, labeling drug candidates with radioactive isotopes can give information about the drug distribution, drug target, and drug metabolism in vivo. In addition, it can also show whether a drug is able to cross specific natural barriers, such as the BBB. In vivo imaging can help to select candidates that appear successful or to discard drugs that seem likely to fail.546
On the other hand, drug candidates can also be used to develop new radiotracers (e.g., PET tracers) targeting ABCA transporters that could then be used in clinical diagnostics. Radiotracers would facilitate the study of the specific gene and/or its product protein in human patients in vivo and in a longitudinal fashion, enabling a much better understanding of the role of ABCA transporters in human (patho)physiology.547 In this regard, knock-out animals can be used as negative controls for the development of new ABCA radiotracers to evaluate the specificity of the radiotracer.548 Furthermore, these very same radiotracers can be used in animal disease models, enabling longitudinal studies and reducing the number of animals required.549-551
CONCLUDING REMARKS: WHERE DO WE GO FROM HERE?
Several in vivo studies demonstrated that modulators of ABCA transporters, in particular ABCA1, have systemic effects.231,249,250,253,271,275,289,293,297,330,335,344,350,361,362,366,368-370,376,378,383,410,415,417-419,425,431,436,448 However, the vast majority of these modulators were regulators,231,250,253,271,297,330,335,344,350,361,362,366,368-370,
376,378,383,410,415,417-419,425,431,436,448 specifically inducers,250,253,271,297,330,344,361,362,366,368-370,376,378,383,410,
415,418,419,425,431 and only very few interactors demonstrated in vivo effects.249,289,293 Mostly emphasizing atherosclerosis,249,275,289,366,369,370,378,410,417-419,425,431,448 these regulators were able to demonstrate that cellular and plasma lipid content249,271,275,289,330,366,369,378,419,425,431 as well as atherosclerotic plaque formation275,289,366,369,370,410, 417-419,425,448 could be changed compared to controls (enhanced or reduced) after treatment with the respective drug. Only very few in vivo approaches targeted for AD.293,297,344,383
Taking the challenge of CNS penetration of these drugs into account, drugs active in atherosclerosis models could generally be suggested to also have certain therapeutic relevance regarding AD. Nevertheless, so far, none of these drugs has made it into clinical evaluation in humans. The underlying cause can be pinned to the fact that the principal mechanism by which ABCA transporters contribute to AD is still unknown. While a rationale can be found in atherosclerosis (efflux of cellular lipid to APOE and HDL resulting in lower lipid burden in the vascular system), the translation of this rationale to AD can only be achieved to a very limited extent. Several questions need addressing in future evaluations: (i) what is the general function of ABCA transporters in the brain to ameliorate (or exacerbate) AD in patients; (ii) when does this development start; and (iii) at which stage of development can a pharmacological intervention with ABCA transporter modulators lead to a positive therapeutic effect?
In this regard, more in vitro tests are needed with new lead structures that are rigorously assessed for their particular mechanism of action – to study vice versa the mechanism of action of ABCA transporters in general. One possibility to gain novel lead structures is the screening of huge analog compound libraries. However, the number of existing compounds is limited, and blind in vitro testing is resource-consuming, especially regarding time and funds. Computational methodologies may help to generate novel lead structures based on the knowledge of existing modulators of ABCA transporters. This has led to new lead molecules in the past.18,19,493,495 Particularly the knowledge on ABCA1 and ABCA8 inhibitors and substrates is of interest, because these compounds inherit the molecular-structural information that is critical for direct binding to these transporters. Considering the newly developed pattern analysis methodology, C@PA,18,19,495 the scaffolds and substructural composition of this set of molecules may reveal the critical necessities for direct interaction with ABCA transporters. C@PA is therefore of high relevance because it was specifically developed to gain multitargeting pan-ABC transporter modulators18,19,495 – molecules that particularly interact with different ABC transporters of different subfamilies. Assuming that a conserved multitarget binding site exists as proposed earlier,6,14,475 multitargeting may be the key to explore under-studied ABC transporters in general and ABCA transporters in particular.6,14,18,19 Several thousands of these molecules have already been predicted,18,19,493,495 and the predictions were in part biologically confirmed.18,19,493,495 Additionally, selected pan-ABC transporter inhibitors were analyzed in molecular docking studies, which revealed the potential existence of the multitarget binding site.475 Hence, combining the existent knowledge of ABCA transporter modulators with (sub)structural elements of these pan-ABC transporter modulators and powerful computational approaches (e.g., molecular docking or molecular dynamics simulations) could ultimately lead to the successful exploration of ABCA transporters in general, as well as ABCA1 and ABCA7 in particular.28,95,103-112
Several drugs and drug-like compounds have already been demonstrated to be pan-ABC transporter modulators interacting also with ABCA transporters. These drugs and drug-like compounds are, for example, cyclosporine A (9 targets of 4 subfamilies: ABCA1,245 ABCB1,20 ABCB4,552 ABCB11,553 ABCC1–2,24,554 ABCC10,26 and ABCG1–2555,556), glibenclamide (8 targets of 4 subfamilies: ABCA1,270 ABCB11,553 ABCC1,24 ABCC5,557 ABCC7–9,558-560 and ABCG2554), imatinib (6 targets of 4 subfamilies: ABCA3,426 ABCB1,561 ABCB11,553 ABCC1,561 ABCC10,561 and ABCG2561), probenecid (8 targets of 2 subfamilies: ABCA8,222 ABCC1–6,24,26,562-564 ABCC10565), verapamil (9 targets of 4 subfamilies: ABCA8,222 ABCB1,20 ABCB4–5,552,566 ABCB11,567 ABCC1,24 ABCC4,568 ABCC10,565 and ABCG2554), and verlukast (11 targets of 4 subfamilies: ABCA8,222 ABCB4,552 ABCB11,553 ABCC1–5,24,554,557,564,569 ABCC10–11,26,570 ABCG2554). In silico analyses with verapamil and verlukast supported the notion of addressing the multitarget binding site in ABCA7.475 Taking their structural peculiarities in a pattern-based rational drug design approach into account may yield novel lead structures for functional in vitro studies of ABCA transporters. This may ultimately result in the development of innovative AD diagnostics and therapeutics.
APPENDIX
Author information
Corresponding author
Sven Marcel Stefan, Department of Pathology, Section of Neuropathology, Translational Neurodegeneration Research and Neuropathology Lab, University of Oslo and Oslo University Hospital, Sognsvannsveien 20, 0372 Oslo, Norway;
ORCiD: 0000-0002-2048-8598
Email: s.m.stefan@medisin.uio.no
Authors
Jens Pahnke, Department of Pathology, Section of Neuropathology, Translational Neurodegeneration Research and Neuropathology Lab, University of Oslo and Oslo University Hospital, Sognsvannsveien 20, 0372 Oslo, Norway; LIED, University of Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany; Department of Pharmacology, Faculty of Medicine, University of Latvia, Jelgavas iela 1, 1004 Rīga, Latvia;
ORCiD: 0000-0001-7355-4213
Pablo Bascuñana, Department of Pathology, Section of Neuropathology, Translational Neurodegeneration Research and Neuropathology Lab, University of Oslo and Oslo University Hospital, Sognsvannsveien 20, 0372 Oslo, Norway;
ORCiD: 0000-0003-2186-8899
Mirjam Brackhan, Department of Pathology, Section of Neuropathology, Translational Neurodeg-eneration Research and Neuropathology Lab, University of Oslo and Oslo University Hospital, Sognsvannsveien 20, 0372 Oslo, Norway; LIED, University of Lübeck, Ratzenburger Allee 160, 23538 Lübeck, Germany;
ORCiD: 0000-0002-0753-6292
Katja Stefan, Department of Pathology, Section of Neuropathology, Translational Neurodegeneration Research and Neuropathology Lab, University of Oslo and Oslo University Hospital, Sognsvannsveien 20, 0372 Oslo, Norway;
ORCiD: 0000-0003-3544-2477
Vigneshwaran Namasivayam, Department of Pharmaceutical and Cellbiological Chemistry, Pharmaceutical Institute, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany;
ORCiD: 0000-0003-3031-3377
Radosveta Koldamova, Department of Environmental and Occupational Health, School of Public Health, University of Pittsburgh, 130 De Soto Street, Pittsburgh, PA 15261, United States of America;
ORCiD: 0000-0002-6761-0984
Jingyun Wu, Department of Pathology, Section of Neuropathology, Translational Neurodegeneration Research and Neuropathology Lab, University of Oslo and Oslo University Hospital, Sognsvannsveien 20, 0372 Oslo, Norway;
ORCiD: 0000-0002-5137-4614
Luisa Möhle, Department of Pathology, Section of Neuropathology, Translational Neurodege-neration Research and Neuropathology Lab, University of Oslo and Oslo University Hospital, Sognsvannsveien 20, 0372 Oslo, Norway;
ORCiD: 0000-0002-4535-9952
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
JP received funding from Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; Germany; 263024513); Latvian Council of Science (Latvia; LZP-2018/1-0275); HelseSØ (Norway; 2019054, 2019055); Barnekreftforeningen (Norway; 19008); EEA grant/Norway grants Kappa programme (Iceland, Liechtenstein, Norway; TAČR TARIMAD TO100078); Norges forskningsråd [Norway; 260786 (PROP-AD), 295910 (NAPI), and 327571 (PETABC)]; European Commission (European Union; 643417).
PROP-AD and PETABC are EU Joint Programme - Neurodegenerative Disease Research (JPND) projects. PROP-AD is supported through the following funding organizations under the aegis of JPND – www.jpnd.eu: AKA #301228 – Finland, BMBF #01ED1605 – Germany; CSO-MOH #30000-12631 – Israel; NFR #260786 – Norway; SRC #2015-06795 – Sweden). PETABC is supported through the following funding organizations under the aegis of JPND – www.jpnd.eu: NFR #327571 – Norway; FFG #882717 – Austria; BMBF #01ED2106 – Germany; MSMT #8F21002 – Czech Republic; VIAA #ES RTD/2020/26 – Latvia; ANR #20-JPW2-0002-04 – France, SRC #2020-02905 – Sweden. The projects receive funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement #643417 (JPco-fuND).
KS receives a Walter Benjamin fellowship of the DFG (Germany; 466106904).
RK is funded by the National Institute of Health (NIH; United States; AG056371, AG057565; AG066198).
LM is supported by the Norwegian Health Association (Nasjonalforeningen for folkehelsen; Norway; #16154).
SMS receives a Walter Benjamin fellowship of the DFG (Germany; 446812474).
Acknowledgement
The authors would like to cordially thank Joseph Mark Robertson (NAPI / Department of Immunology, University of Oslo and Oslo University Hospital) for proofreading the manuscript.
References
- Wang, J. Q.; Yang, Y.; Cai, C. Y.; Teng, Q. X.; Cui, Q.; Lin, J.; Assaraf, Y. G.; Chen, Z. S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist Updat 2021, 54, 100743.
- Gil-Martins, E.; Barbosa, D. J.; Silva, V.; Remiao, F.; Silva, R. Dysfunction of ABC transporters at the blood-brain barrier: Role in neurological disorders. Pharmacol Ther 2020, 213, 107554.
- Pasello, M.; Giudice, A. M.; Scotlandi, K. The ABC subfamily A transporters: Multifaceted players with incipient potentialities in cancer. Semin Cancer Biol 2020, 60, 57-71.
- Sodani, K.; Patel, A.; Kathawala, R. J.; Chen, Z. S. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer 2012, 31, 58-72.
- Szakacs, G.; Abele, R. An inventory of lysosomal ABC transporters. FEBS Lett 2020, 594, 3965-85.
- Stefan, K.; Leck, L. Y. W.; Namasivayam, V.; Bascuñana, P.; Huang, M. L.-H.; Riss, P. J.; Pahnke, J.; Jansson, P. J.; Stefan, S. M. Vesicular ATP-binding cassette transporters in human disease: relevant aspects of their organization for future drug development. Future Drug Discov 2020, 2, FDD51.
- Domenichini, A.; Adamska, A.; Falasca, M. ABC transporters as cancer drivers: Potential functions in cancer development. Biochim Biophys Acta Gen Subj 2019, 1863, 52-60.
- Adamska, A.; Falasca, M. ATP-binding cassette transporters in progression and clinical outcome of pancreatic cancer: What is the way forward? World J Gastroenterol 2018, 24, 3222-38.
- Robey, R. W.; Pluchino, K. M.; Hall, M. D.; Fojo, A. T.; Bates, S. E.; Gottesman, M. M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 2018, 18, 452-64.
- Sasaki, K.; Tachikawa, M.; Uchida, Y.; Hirano, S.; Kadowaki, F.; Watanabe, M.; Ohtsuki, S.; Terasaki, T. ATP-binding cassette transporter a subfamily 8 is a sinusoidal efflux transporter for cholesterol and taurocholate in mouse and human liver. Mol Pharm 2018, 15, 343-55.
- Pan, S. T.; Li, Z. L.; He, Z. X.; Qiu, J. X.; Zhou, S. F. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol 2016, 43, 723-37.
- Ween, M. P.; Armstrong, M. A.; Oehler, M. K.; Ricciardelli, C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit Rev Oncol Hematol 2015, 96, 220-56.
- Wenzel, J. J.; Piehler, A.; Kaminski, W. E. ABC A-subclass proteins: gatekeepers of cellular phospho- and sphingolipid transport. Front Biosci 2007, 12, 3177-93.
- Stefan, S. M. Multi-target ABC transporter modulators: what next and where to go? Future Med Chem 2019, 11, 2353-58.
- Yu, M.; Ocana, A.; Tannock, I. F. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer Metastasis Rev 2013, 32, 211-27.
- Amiri-Kordestani, L.; Basseville, A.; Kurdziel, K.; Fojo, A. T.; Bates, S. E. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat 2012, 15, 50-61.
- Tamaki, A.; Ierano, C.; Szakacs, G.; Robey, R. W.; Bates, S. E. The controversial role of ABC transporters in clinical oncology. Essays Biochem 2011, 50, 209-32.
- Namasivayam, V.; Silbermann, K.; Pahnke, J.; Wiese, M.; Stefan, S. M. Scaffold fragmentation and substructure hopping reveal potential, robustness, and limits of computer-aided pattern analysis (C@PA). Comput Struct Biotechnol J 2021, 19, 3269-83.
- Namasivayam, V.; Silbermann, K.; Wiese, M.; Pahnke, J.; Stefan, S. M. C@PA: computer-aided pattern analysis to predict multitarget ABC transporter inhibitors. J Med Chem 2021, 64, 3350-66.
- Zhang, H.; Xu, H.; Ashby, C. R., Jr.; Assaraf, Y. G.; Chen, Z. S.; Liu, H. M. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med Res Rev 2021, 41, 525-55.
- Dong, J.; Qin, Z.; Zhang, W. D.; Cheng, G.; Yehuda, A. G.; Ashby, C. R., Jr.; Chen, Z. S.; Cheng, X. D.; Qin, J. J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist Updat 2020, 49, 100681.
- Palmeira, A.; Sousa, E.; Vasconcelos, M. H.; Pinto, M. M. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem 2012, 19, 1946-2025.
- Wiese, M.; Stefan, S. M. The A-B-C of small-molecule ABC transport protein modulators: From inhibition to activation-a case study of multidrug resistance-associated protein 1 (ABCC1). Med Res Rev 2019, 39, 2031-81.
- Stefan, S. M.; Wiese, M. Small-molecule inhibitors of multidrug resistance-associated protein 1 and related processes: A historic approach and recent advances. Med Res Rev 2019, 39, 176-264.
- Pena-Solorzano, D.; Stark, S. A.; Konig, B.; Sierra, C. A.; Ochoa-Puentes, C. ABCG2/BCRP: specific and nonspecific modulators. Med Res Rev 2017, 37, 987-1050.
- Zhou, S. F.; Wang, L. L.; Di, Y. M.; Xue, C. C.; Duan, W.; Li, C. G.; Li, Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem 2008, 15, 1981-2039.
- Norman, B. H. Inhibitors of MRP1-mediated multidrug resistance. Drugs Fut 1998, 23(9), 1001.
- Pereira, C. D.; Martins, F.; Wiltfang, J.; da Cruz, E. S. O. A. B.; Rebelo, S. ABC transporters are key players in alzheimer's disease. J Alzheimers Dis 2018, 61, 463-85.
- Abuznait, A. H.; Kaddoumi, A. Role of ABC transporters in the pathogenesis of Alzheimer's disease. ACS Chem Neurosci 2012, 3, 820-31.
- Wolf, A.; Bauer, B.; Hartz, A. M. ABC Transporters and the Alzheimer's Disease Enigma. Front Psychiatry 2012, 3, 54.
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 2011, 8, 3.
- Kortekaas, R.; Leenders, K. L.; van Oostrom, J. C.; Vaalburg, W.; Bart, J.; Willemsen, A. T.; Hendrikse, N. H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 2005, 57, 176-9.
- Jha, N. K.; Kar, R.; Niranjan, R. ABC transporters in neurological disorders: An important gateway for botanical compounds mediated neuro-therapeutics. Curr Top Med Chem 2019, 19, 795-811.
- Jablonski, M. R.; Markandaiah, S. S.; Jacob, D.; Meng, N. J.; Li, K.; Gennaro, V.; Lepore, A. C.; Trotti, D.; Pasinelli, P. Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann Clin Transl Neurol 2014, 1, 996-1005.
- Kooij, G.; Kroon, J.; Paul, D.; Reijerkerk, A.; Geerts, D.; van der Pol, S. M.; van Het Hof, B.; Drexhage, J. A.; van Vliet, S. J.; Hekking, L. H.; van Buul, J. D.; Pachter, J. S.; de Vries, H. E. P-glycoprotein regulates trafficking of CD8(+) T cells to the brain parenchyma. Acta Neuropathol 2014, 127, 699-711.
- Jablonski, M. R.; Jacob, D. A.; Campos, C.; Miller, D. S.; Maragakis, N. J.; Pasinelli, P.; Trotti, D. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol Dis 2012, 47, 194-200.
- Westerlund, M.; Belin, A. C.; Olson, L.; Galter, D. Expression of multi-drug resistance 1 mRNA in human and rodent tissues: reduced levels in Parkinson patients. Cell Tissue Res 2008, 334, 179-85.
- Valenza, M.; Carroll, J. B.; Leoni, V.; Bertram, L. N.; Bjorkhem, I.; Singaraja, R. R.; Di Donato, S.; Lutjohann, D.; Hayden, M. R.; Cattaneo, E. Cholesterol biosynthesis pathway is disturbed in YAC128 mice and is modulated by huntingtin mutation. Hum Mol Genet 2007, 16, 2187-98.
- Dombrowski, S. M.; Desai, S. Y.; Marroni, M.; Cucullo, L.; Goodrich, K.; Bingaman, W.; Mayberg, M. R.; Bengez, L.; Janigro, D. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 2001, 42, 1501-6.
- Sisodiya, S. M.; Lin, W. R.; Harding, B. N.; Squier, M. V.; Thom, M. Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain 2002, 125, 22-31.
- Katzeff, J. S.; Kim, W. S. ATP-binding cassette transporters and neurodegenerative diseases. Essays Biochem 2021 EBC20210012.
- Dinda, B.; Dinda, M.; Kulsi, G.; Chakraborty, A.; Dinda, S. Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review. Eur J Med Chem 2019, 169, 185-99.
- Pahnke, J.; Langer, O.; Krohn, M. Alzheimer's and ABC transporters--new opportunities for diagnostics and treatment. Neurobiol Dis 2014, 72 Pt A, 54-60.
- Susa, M.; Iyer, A. K.; Ryu, K.; Choy, E.; Hornicek, F. J.; Mankin, H.; Milane, L.; Amiji, M. M.; Duan, Z. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PloS one 2010, 5, e10764.
- Robillard, K. R.; Hoque, M. T.; Bendayan, R. Expression of ATP-binding cassette membrane transporters in a HIV-1 transgenic rat model. Biochem Biophys Res Commun 2014, 444, 531-6.
- Hayashi, K.; Pu, H.; Tian, J.; Andras, I. E.; Lee, Y. W.; Hennig, B.; Toborek, M. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem 2005, 93, 1231-41.
- Matsuo, H.; Tomiyama, H.; Satake, W.; Chiba, T.; Onoue, H.; Kawamura, Y.; Nakayama, A.; Shimizu, S.; Sakiyama, M.; Funayama, M.; Nishioka, K.; Shimizu, T.; Kaida, K.; Kamakura, K.; Toda, T.; Hattori, N.; Shinomiya, N. ABCG2 variant has opposing effects on onset ages of Parkinson's disease and gout. Ann Clin Transl Neurol 2015, 2, 302-6.
- Nakato, M.; Shiranaga, N.; Tomioka, M.; Watanabe, H.; Kurisu, J.; Kengaku, M.; Komura, N.; Ando, H.; Kimura, Y.; Kioka, N.; Ueda, K. ABCA13 dysfunction associated with psychiatric disorders causes impaired cholesterol trafficking. J Biol Chem 2020, 296, 100166.
- Dwyer, S.; Williams, H.; Jones, I.; Jones, L.; Walters, J.; Craddock, N.; Owen, M. J.; O'Donovan, M. C. Investigation of rare non-synonymous variants at ABCA13 in schizophrenia and bipolar disorder. Mol Psychiatry 2011, 16, 790-1.
- Nordestgaard, L. T.; Tybjaerg-Hansen, A.; Nordestgaard, B. G.; Frikke-Schmidt, R. Loss-of-function mutation in ABCA1 and risk of Alzheimer's disease and cerebrovascular disease. Alzheimers Dement 2015, 11, 1430-8.
- Gonzalez-Guevara, E.; Cardenas, G.; Perez-Severiano, F.; Martinez-Lazcano, J. C. dysregulated brain cholesterol metabolism is linked to neuroinflammation in Huntington's disease. Mov Disord 2020, 35, 1113-27.
- Macé, S.; Cousin, E.; Ricard, S.; Génin, E.; Spanakis, E.; Lafargue-Soubigou, C.; Génin, B.; Fournel, R.; Roche, S.; Haussy, G.; Massey, F.; Soubigou, S.; Bréfort, G.; Benoit, P.; Brice, A.; Campion, D.; Hollis, M.; Pradier, L.; Benavides, J.; Deleuze, J. F. ABCA2 is a strong genetic risk factor for early-onset Alzheimer's disease. Neurobiol Dis 2005, 18, 119-25.
- Davis, W., Jr. The ATP-binding cassette transporter-2 (ABCA2) regulates esterification of plasma membrane cholesterol by modulation of sphingolipid metabolism. Biochim Biophys Acta 2014, 1841, 168-79.
- Sakai, H.; Tanaka, Y.; Tanaka, M.; Ban, N.; Yamada, K.; Matsumura, Y.; Watanabe, D.; Sasaki, M.; Kita, T.; Inagaki, N. ABCA2 deficiency results in abnormal sphingolipid metabolism in mouse brain. J Biol Chem 2007, 282, 19692-9.
- Maugeri, A.; Klevering, B. J.; Rohrschneider, K.; Blankenagel, A.; Brunner, H. G.; Deutman, A. F.; Hoyng, C. B.; Cremers, F. P. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet 2000, 67, 960-6.
- Cremers, F. P. M.; Lee, W.; Collin, R. W. J.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog Retin Eye Res 2020, 79, 100861.
- Cremers, F. P.; van de Pol, D. J.; van Driel, M.; den Hollander, A. I.; van Haren, F. J.; Knoers, N. V.; Tijmes, N.; Bergen, A. A.; Rohrschneider, K.; Blankenagel, A.; Pinckers, A. J.; Deutman, A. F.; Hoyng, C. B. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet 1998, 7, 355-62.
- Martínez-Mir, A.; Paloma, E.; Allikmets, R.; Ayuso, C.; del Rio, T.; Dean, M.; Vilageliu, L.; Gonzàlez-Duarte, R.; Balcells, S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet 1998, 18, 11-2.
- Srisuwanwattana, P.; Vachiramon, V. Necrolytic Acral Erythema in Seronegative Hepatitis C. Case Rep Dermatol 2017, 9, 69-73.
- Conley, S. M.; Cai, X.; Makkia, R.; Wu, Y.; Sparrow, J. R.; Naash, M. I. Increased cone sensitivity to ABCA4 deficiency provides insight into macular vision loss in Stargardt's dystrophy. Biochim Biophys Acta 2012, 1822, 1169-79.
- Cideciyan, A. V.; Aleman, T. S.; Swider, M.; Schwartz, S. B.; Steinberg, J. D.; Brucker, A. J.; Maguire, A. M.; Bennett, J.; Stone, E. M.; Jacobson, S. G. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum Mol Genet 2004, 13, 525-34.
- Allikmets, R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 1997, 17, 122.
- Kjeldsen, E. W.; Tybjaerg-Hansen, A.; Nordestgaard, B. G.; Frikke-Schmidt, R. ABCA7 and risk of dementia and vascular disease in the Danish population. Ann Clin Transl Neurol 2018, 5, 41-51.
- Rajkumar, A. P.; Bidkhori, G.; Shoaie, S.; Clarke, E.; Morrin, H.; Hye, A.; Williams, G.; Ballard, C.; Francis, P.; Aarsland, D. postmortem cortical transcriptomics of Lewy body dementia reveal mitochondrial dysfunction and lack of neuroinflammation. Am J Geriatr Psychiatry 2020, 28, 75-86.
- Qian, L.; Qin, Y.; Chen, X.; Zhang, F.; Yang, B.; Dong, K.; Wang, Z.; Zhang, K. ATP-binding cassette transporter 13 mRNA expression level in schizophrenia patients. Sci Rep 2020, 10, 21498.
- Crespo-Facorro, B.; Prieto, C.; Sainz, J. Schizophrenia gene expression profile reverted to normal levels by antipsychotics. Int J Neuropsychopharmacol 2014, 18, pyu066.
- Dongsheng, H.; Zhuo, Z.; Jiamin, L.; Hailan, M.; Lijuan, H.; Fan, C.; Dan, Y.; He, Z.; Yun, X. proteomic analysis of the peri-infarct area after human umbilical cord mesenchymal stem cell transplantation in experimental stroke. Aging Dis 2016, 7, 623-34.
- Ginguene, C.; Champier, J.; Maallem, S.; Strazielle, N.; Jouvet, A.; Fevre-Montange, M.; Ghersi-Egea, J. F. P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) localize in the microvessels forming the blood-tumor barrier in ependymomas. Brain Pathol 2010, 20, 926-35.
- Bernstein, H. G.; Hildebrandt, J.; Dobrowolny, H.; Steiner, J.; Bogerts, B.; Pahnke, J. Morphometric analysis of the cerebral expression of ATP-binding cassette transporter protein ABCB1 in chronic schizophrenia: Circumscribed deficits in the habenula. Schizophr Res 2016, 177, 52-58.
- de Klerk, O. L.; Willemsen, A. T.; Roosink, M.; Bartels, A. L.; Hendrikse, N. H.; Bosker, F. J.; den Boer, J. A. Locally increased P-glycoprotein function in major depression: a PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood-brain barrier. Int J Neuropsychopharmacol 2009, 12, 895-904.
- Seegers, U.; Potschka, H.; Loscher, W. Transient increase of P-glycoprotein expression in endothelium and parenchyma of limbic brain regions in the kainate model of temporal lobe epilepsy. Epilepsy Res 2002, 51, 257-68.
- Patak, P.; Hermann, D. M. ATP-binding cassette transporters at the blood-brain barrier in ischaemic stroke. Curr Pharm Des 2011, 17, 2787-92.
- Bartels, A. L.; Willemsen, A. T.; Kortekaas, R.; de Jong, B. M.; de Vries, R.; de Klerk, O.; van Oostrom, J. C.; Portman, A.; Leenders, K. L. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson's disease, PSP and MSA. J Neural Transm (Vienna) 2008, 115, 1001-9.
- Vautier, S.; Fernandez, C. ABCB1: the role in Parkinson's disease and pharmacokinetics of antiparkinsonian drugs. Expert Opin Drug Metab Toxicol 2009, 5, 1349-58.
- Bernstein, H. G.; Holzl, G.; Dobrowolny, H.; Hildebrandt, J.; Trubner, K.; Krohn, M.; Bogerts, B.; Pahnke, J. Vascular and extravascular distribution of the ATP-binding cassette transporters ABCB1 and ABCC1 in aged human brain and pituitary. Mech Ageing Dev 2014, 141-142, 12-21.
- Vogelgesang, S.; Glatzel, M.; Walker, L. C.; Kroemer, H. K.; Aguzzi, A.; Warzok, R. W. Cerebrovascular P-glycoprotein expression is decreased in Creutzfeldt-Jakob disease. Acta Neuropathol 2006, 111, 436-43.
- Chi, H.; Tang, W.; Bai, Y. Molecular evidence of impaired iron metabolism and its association with Parkinson's disease progression. 3 Biotech 2020, 10, 173.
- Chuang, Y. H.; Paul, K. C.; Bronstein, J. M.; Bordelon, Y.; Horvath, S.; Ritz, B. Parkinson's disease is associated with DNA methylation levels in human blood and saliva. Genome Med 2017, 9, 76.
- Decleves, X.; Fajac, A.; Lehmann-Che, J.; Tardy, M.; Mercier, C.; Hurbain, I.; Laplanche, J. L.; Bernaudin, J. F.; Scherrmann, J. M. Molecular and functional MDR1-Pgp and MRPs expression in human glioblastoma multiforme cell lines. Int J Cancer. 2002, 98, 173-80.
- Kilic, E.; Spudich, A.; Kilic, U.; Rentsch, K. M.; Vig, R.; Matter, C. M.; Wunderli-Allenspach, H.; Fritschy, J. M.; Bassetti, C. L.; Hermann, D. M. ABCC1: a gateway for pharmacological compounds to the ischaemic brain. Brain 2008, 131, 2679-89.
- Vidal-Taboada, J. M.; Pugliese, M.; Salvado, M.; Gamez, J.; Mahy, N.; Rodriguez, M. J. KATP channel expression and genetic polymorphisms associated with progression and survival in amyotrophic lateral sclerosis. Mol Neurobiol 2018, 55, 7962-72.
- Crary, J. F. Top ten discoveries of the year: Neurodegeneration. Free Neuropathol 2020, 1, 12.
- Nelson, P. T.; Jicha, G. A.; Wang, W. X.; Ighodaro, E.; Artiushin, S.; Nichols, C. G.; Fardo, D. W. ABCC9/SUR2 in the brain: Implications for hippocampal sclerosis of aging and a potential therapeutic target. Ageing Res Rev 2015, 24, 111-25.
- Berger, J.; Forss-Petter, S.; Eichler, F. S. Pathophysiology of X-linked adrenoleukodystrophy. Biochim 2014, 98, 135-42.
- Tansley, G. H.; Burgess, B. L.; Bryan, M. T.; Su, Y.; Hirsch-Reinshagen, V.; Pearce, J.; Chan, J. Y.; Wilkinson, A.; Evans, J.; Naus, K. E.; McIsaac, S.; Bromley, K.; Song, W.; Yang, H. C.; Wang, N.; DeMattos, R. B.; Wellington, C. L. The cholesterol transporter ABCG1 modulates the subcellular distribution and proteolytic processing of beta-amyloid precursor protein. J Lipid Res 2007, 48, 1022-34.
- Burgess, B. L.; Parkinson, P. F.; Racke, M. M.; Hirsch-Reinshagen, V.; Fan, J.; Wong, C.; Stukas, S.; Theroux, L.; Chan, J. Y.; Donkin, J.; Wilkinson, A.; Balik, D.; Christie, B.; Poirier, J.; Lutjohann, D.; Demattos, R. B.; Wellington, C. L. ABCG1 influences the brain cholesterol biosynthetic pathway but does not affect amyloid precursor protein or apolipoprotein E metabolism in vivo. J Lipid Res 2008, 49, 1254-67.
- Shen, S.; Callaghan, D.; Juzwik, C.; Xiong, H.; Huang, P.; Zhang, W. ABCG2 reduces ROS-mediated toxicity and inflammation: a potential role in Alzheimer's disease. J Neurochem 2010, 114, 1590-604.
- van Vliet, E. A.; Iyer, A. M.; Mesarosova, L.; Colakoglu, H.; Anink, J. J.; van Tellingen, O.; Maragakis, N. J.; Shefner, J.; Bunt, T.; Aronica, E. Expression and cellular distribution of p-glycoprotein and breast cancer resistance protein in amyotrophic lateral sclerosis patients. J Neuropathol Exp Neurol 2020, 79, 266-276.
- Bleau, A. M.; Hambardzumyan, D.; Ozawa, T.; Fomchenko, E. I.; Huse, J. T.; Brennan, C. W.; Holland, E. C. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 2009, 4, 226-35.
- van Vliet, E. A.; Redeker, S.; Aronica, E.; Edelbroek, P. M.; Gorter, J. A. Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia 2005, 46, 1569-80.
- Kooij, G.; Mizee, M. R.; van Horssen, J.; Reijerkerk, A.; Witte, M. E.; Drexhage, J. A.; van der Pol, S. M.; van Het Hof, B.; Scheffer, G.; Scheper, R.; Dijkstra, C. D.; van der Valk, P.; de Vries, H. E. Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 2011, 134, 555-70.
- Adams, S. M.; Conley, Y. P.; Ren, D.; Okonkwo, D. O.; Puccio, A. M.; Dixon, C. E.; Clark, R. S. B.; Kochanek, P. M.; Empey, P. E. ABCG2 c.421C>A Is Associated with Outcomes after Severe Traumatic Brain Injury. J Neurotrauma 2018, 35, 48-53.
- Do, T. M.; Noel-Hudson, M. S.; Ribes, S.; Besengez, C.; Smirnova, M.; Cisternino, S.; Buyse, M.; Calon, F.; Chimini, G.; Chacun, H.; Scherrmann, J. M.; Farinotti, R.; Bourasset, F. ABCG2- and ABCG4-mediated efflux of amyloid-beta peptide 1-40 at the mouse blood-brain barrier. J Alzheimers Dis 2012, 30, 155-66.
- Lam, F. C.; Liu, R.; Lu, P.; Shapiro, A. B.; Renoir, J. M.; Sharom, F. J.; Reiner, P. B. beta-Amyloid efflux mediated by p-glycoprotein. J Neurochem 2001, 76, 1121-8.
- Piehler, A. P.; Ozcurumez, M.; Kaminski, W. E. A-Subclass ATP-binding cassette proteins in brain lipid homeostasis and neurodegeneration. Front Psychiatry 2012, 3, 17.
- Krohn, M.; Lange, C.; Hofrichter, J.; Scheffler, K.; Stenzel, J.; Steffen, J.; Schumacher, T.; Bruning, T.; Plath, A. S.; Alfen, F.; Schmidt, A.; Winter, F.; Rateitschak, K.; Wree, A.; Gsponer, J.; Walker, L. C.; Pahnke, J. Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest 2011, 121, 3924-31.
- Xiong, H.; Callaghan, D.; Jones, A.; Bai, J.; Rasquinha, I.; Smith, C.; Pei, K.; Walker, D.; Lue, L. F.; Stanimirovic, D.; Zhang, W. ABCG2 is upregulated in Alzheimer's brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1-40) peptides. J Neurosci 2009, 29, 5463-75.
- Holmes, C.; Boche, D.; Wilkinson, D.; Yadegarfar, G.; Hopkins, V.; Bayer, A.; Jones, R. W.; Bullock, R.; Love, S.; Neal, J. W.; Zotova, E.; Nicoll, J. A. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008, 372, 216-23.
- Pahnke, J.; Walker, L. C.; Scheffler, K.; Krohn, M. Alzheimer's disease and blood-brain barrier function-Why have anti-beta-amyloid therapies failed to prevent dementia progression? Neurosci Biobehav Rev 2009, 33, 1099-108.
- Pahnke, J.; Wolkenhauer, O.; Krohn, M.; Walker, L. C. Clinico-pathologic function of cerebral ABC transporters - implications for the pathogenesis of Alzheimer's disease. Curr Alzheimer Res 2008, 5, 396-405.
- Walker, L. C. Abeta plaques. Free Neuropathol 2020, 1, 31.
- Behl, T.; Kaur, I.; Sehgal, A.; Kumar, A.; Uddin, M. S.; Bungau, S. The Interplay of ABC transporters in Abeta translocation and cholesterol metabolism: Implicating their roles in Alzheimer's disease. Mol Neurobiol 2021, 58, 1564-82.
- Koldamova, R.; Fitz, N. F.; Lefterov, I. ATP-binding cassette transporter A1: from metabolism to neurodegeneration. Neurobiol Dis 2014, 72 Pt A, 13-21.
- Lyssenko, N. N.; Praticò, D. ABCA7 and the altered lipidostasis hypothesis of Alzheimer's disease. Alzheimers Dement 2020, 17, 164-174.
- Abe-Dohmae, S.; Yokoyama, S. ABCA7 links sterol metabolism to the host defense system: Molecular background for potential management measure of Alzheimer's disease. Gene 2021, 768, 145316.
- Aikawa, T.; Holm, M. L.; Kanekiyo, T. ABCA7 and Pathogenic Pathways of Alzheimer's Disease. Brain Sci 2018, 8, 27.
- Li, H.; Karl, T.; Garner, B. Understanding the function of ABCA7 in Alzheimer's disease. Biochem Soc Trans 2015, 43, 920-3.
- Andrews, S. J.; Fulton-Howard, B.; Goate, A. protective variants in Alzheimer's disease. Curr Genet Med Rep 2019, 7, 1-12.
- Teresa, J. C.; Fernado, C.; Nancy, M. R.; Gilberto, V. A.; Alberto, C. R.; Roberto, R. R. Association of genetic variants of ABCA1 with susceptibility to dementia: (SADEM study). Metab Brain Dis 2020, 35, 915-22.
- Jiang, S.; Zhang, C. Y.; Tang, L.; Zhao, L. X.; Chen, H. Z.; Qiu, Y. integrated genomic analysis revealed associated genes for Alzheimer's disease in APOE4 non-carriers. Curr Alzheimer Res 2019, 16, 753-63.
- Chen, Q.; Liang, B.; Wang, Z.; Cheng, X.; Huang, Y.; Liu, Y.; Huang, Z. Influence of four polymorphisms in ABCA1 and PTGS2 genes on risk of Alzheimer's disease: a meta-analysis. Neurol Sci 2016, 37, 1209-20.
- Piaceri, I.; Nacmias, B.; Sorbi, S. Genetics of familial and sporadic Alzheimer's disease. Front Biosci (Elite Ed) 2013, 5, 167-77.
- Agarwal, M.; Khan, S. plasma lipids as biomarkers for Alzheimer's disease: A systematic review. Cureus 2020, 12, e12008.
- Picard, C.; Julien, C.; Frappier, J.; Miron, J.; Théroux, L.; Dea, D.; Breitner, J. C. S.; Poirier, J. Alterations in cholesterol metabolism-related genes in sporadic Alzheimer's disease. Neurobiol Aging 2018, 66, 180.e1-180.e9.
- Beel, A. J.; Sakakura, M.; Barrett, P. J.; Sanders, C. R. Direct binding of cholesterol to the amyloid precursor protein: An important interaction in lipid-Alzheimer's disease relationships? Biochim Biophys Acta 2010, 1801, 975-82.
- Shobab, L. A.; Hsiung, G. Y.; Feldman, H. H. Cholesterol in Alzheimer's disease. Lancet Neurol 2005, 4, 841-52.
- Sviridov, D.; Mukhamedova, N.; Miller, Y. I. Lipid rafts as a therapeutic target. J Lipid Res 2020, 61, 687-695.
- Satoh, K.; Abe-Dohmae, S.; Yokoyama, S.; St George-Hyslop, P.; Fraser, P. E. ATP-binding cassette transporter A7 (ABCA7) loss of function alters Alzheimer amyloid processing. J Biol Chem 2015, 290, 24152-65.
- Zhao, Q. F.; Yu, J. T.; Tan, M. S.; Tan, L. ABCA7 in Alzheimer's Disease. Mol Neurobiol 2015, 51, 1008-16.
- Chan, S. L.; Kim, W. S.; Kwok, J. B.; Hill, A. F.; Cappai, R.; Rye, K. A.; Garner, B. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J Neurochem 2008, 106, 793-804.
- Sun, Y.; Yao, J.; Kim, T. W.; Tall, A. R. Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J Biol Chem 2003, 278, 27688-94.
- Koldamova, R. P.; Lefterov, I. M.; Ikonomovic, M. D.; Skoko, J.; Lefterov, P. I.; Isanski, B. A.; DeKosky, S. T.; Lazo, J. S. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem 2003, 278, 13244-56.
- Fu, Y.; Hsiao, J. H.; Paxinos, G.; Halliday, G. M.; Kim, W. S. ABCA7 Mediates Phagocytic Clearance of Amyloid-beta in the Brain. J Alzheimers Dis 2016, 54, 569-84.
- Kim, W. S.; Li, H.; Ruberu, K.; Chan, S.; Elliott, D. A.; Low, J. K.; Cheng, D.; Karl, T.; Garner, B. Deletion of Abca7 increases cerebral amyloid-beta accumulation in the J20 mouse model of Alzheimer's disease. J Neurosci 2013, 33, 4387-94.
- Koldamova, R.; Fitz, N. F.; Lefterov, I. The role of ATP-binding cassette transporter A1 in Alzheimer's disease and neurodegeneration. Biochim Biophys Acta 2010, 1801, 824-30.
- Jiang, Q.; Lee, C. Y.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T. M.; Collins, J. L.; Richardson, J. C.; Smith, J. D.; Comery, T. A.; Riddell, D.; Holtzman, D. M.; Tontonoz, P.; Landreth, G. E. ApoE promotes the proteolytic degradation of Abeta. Neuron 2008, 58, 681-93.
- Wahrle, S. E.; Jiang, H.; Parsadanian, M.; Kim, J.; Li, A.; Knoten, A.; Jain, S.; Hirsch-Reinshagen, V.; Wellington, C. L.; Bales, K. R.; Paul, S. M.; Holtzman, D. M. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest 2008, 118, 671-82.
- Saint-Pol, J.; Vandenhaute, E.; Boucau, M. C.; Candela, P.; Dehouck, L.; Cecchelli, R.; Dehouck, M. P.; Fenart, L.; Gosselet, F. Brain pericytes ABCA1 expression mediates cholesterol efflux but not cellular amyloid-beta peptide accumulation. J Alzheimers Dis 2012, 30, 489-503.
- Akanuma, S.; Ohtsuki, S.; Doi, Y.; Tachikawa, M.; Ito, S.; Hori, S.; Asashima, T.; Hashimoto, T.; Yamada, K.; Ueda, K.; Iwatsubo, T.; Terasaki, T. ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-beta peptide (1-40) at the blood-brain barrier. Neurochem Int 2008, 52, 956-61.
- Albrecht, C.; Viturro, E. The ABCA subfamily--gene and protein structures, functions and associated hereditary diseases. Pflugers Arch 2007, 453, 581-9.
- Kubo, Y.; Sekiya, S.; Ohigashi, M.; Takenaka, C.; Tamura, K.; Nada, S.; Nishi, T.; Yamamoto, A.; Yamaguchi, A. ABCA5 resides in lysosomes, and ABCA5 knockout mice develop lysosomal disease-like symptoms. Mol Cell Biol 2005, 25, 4138-49.
- Takahashi, K.; Kimura, Y.; Nagata, K.; Yamamoto, A.; Matsuo, M.; Ueda, K. ABC proteins: key molecules for lipid homeostasis. Med Mol Morphol 2005, 38, 2-12.
- Garces, F. A.; Scortecci, J. F.; Molday, R. S. Functional characterization of ABCA4 missense variants linked to stargardt macular degeneration. Int J Mol Sci 2020, 22, 185.
- Curtis, S. B.; Molday, L. L.; Garces, F. A.; Molday, R. S. Functional analysis and classification of homozygous and hypomorphic ABCA4 variants associated with Stargardt macular degeneration. Hum Mutat 2020, 41, 1944-1956.
- Garces, F.; Jiang, K.; Molday, L. L.; Stohr, H.; Weber, B. H.; Lyons, C. J.; Maberley, D.; Molday, R. S. Correlating the expression and functional activity of ABCA4 disease variants with the phenotype of patients with stargardt disease. Invest Ophthalmol Vis Sci 2018, 59, 2305-2315.
- Quazi, F.; Lenevich, S.; Molday, R. S. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun 2012, 3, 925.
- Ahn, J.; Wong, J. T.; Molday, R. S. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem 2000, 275, 20399-405.
- Sun, H.; Molday, R. S.; Nathans, J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem 1999, 274, 8269-81.
- Wambach, J. A.; Yang, P.; Wegner, D. J.; Heins, H. B.; Kaliberova, L. N.; Kaliberov, S. A.; Curiel, D. T.; White, F. V.; Hamvas, A.; Hackett, B. P.; Cole, F. S. functional characterization of ATP-binding cassette transporter A3 mutations from infants with respiratory distress syndrome. Am J Respir Cell Mol Biol 2016, 55, 716-21.
- Nathan, N.; Berdah, L.; Delestrain, C.; Sileo, C.; Clement, A. Interstitial lung diseases in children. Presse Med 2020, 49, 103909.
- Chen, P.; Dai, Y.; Wu, X.; Wang, Y.; Sun, S.; Xiao, J.; Zhang, Q.; Guan, L.; Zhao, X.; Hao, X.; Wu, R.; Xie, L. Mutations in the ABCA3 gene are associated with cataract-microcornea syndrome. Invest Ophthalmol Vis Sci 2014, 55, 8031-43.
- DeStefano, G. M.; Kurban, M.; Anyane-Yeboa, K.; Dall'Armi, C.; Di Paolo, G.; Feenstra, H.; Silverberg, N.; Rohena, L.; Lopez-Cepeda, L. D.; Jobanputra, V.; Fantauzzo, K. A.; Kiuru, M.; Tadin-Strapps, M.; Sobrino, A.; Vitebsky, A.; Warburton, D.; Levy, B.; Salas-Alanis, J. C.; Christiano, A. M. Mutations in the cholesterol transporter gene ABCA5 are associated with excessive hair overgrowth. PLoS genetics 2014, 10, e1004333.
- Elkhatib, A. M.; Omar, M. Ichthyosis Fetalis. In StatPearls, Treasure Island (FL), 2021.
- Zarubica, A.; Trompier, D.; Chimini, G. ABCA1, from pathology to membrane function. Pflugers Arch 2007, 453, 569-79.
- Luciani, M. F.; Denizot, F.; Savary, S.; Mattei, M. G.; Chimini, G. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics 1994, 21, 150-9.
- Santamarina-Fojo, S.; Peterson, K.; Knapper, C.; Qiu, Y.; Freeman, L.; Cheng, J. F.; Osorio, J.; Remaley, A.; Yang, X. P.; Haudenschild, C.; Prades, C.; Chimini, G.; Blackmon, E.; Francois, T.; Duverger, N.; Rubin, E. M.; Rosier, M.; Denefle, P.; Fredrickson, D. S.; Brewer, H. B., Jr. Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc Natl Acad Sci U S A 2000, 97, 7987-92.
- Tang, C.; Oram, J. F. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim Biophys Acta 2009, 1791, 563-72.
- Oram, J. F.; Lawn, R. M. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res 2001, 42, 1173-9.
- Hafiane, A.; Gianopoulos, I.; Sorci-Thomas, M. G.; Daskalopoulou, S. S. Current models of apolipoprotein A-I lipidation by adenosine triphosphate binding cassette transporter A1. Curr Opin Lipidol 2021.
- Hirsch-Reinshagen, V.; Zhou, S.; Burgess, B. L.; Bernier, L.; McIsaac, S. A.; Chan, J. Y.; Tansley, G. H.; Cohn, J. S.; Hayden, M. R.; Wellington, C. L. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem 2004, 279, 41197-207.
- Wahrle, S. E.; Jiang, H.; Parsadanian, M.; Legleiter, J.; Han, X.; Fryer, J. D.; Kowalewski, T.; Holtzman, D. M. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem 2004, 279, 40987-93.
- Fitz, N. F.; Carter, A. Y.; Tapias, V.; Castranio, E. L.; Kodali, R.; Lefterov, I.; Koldamova, R. ABCA1 deficiency affects basal cognitive deficits and dendritic density in mice. J Alzheimers Dis 2017, 56, 1075-85.
- Corder, E. H.; Saunders, A. M.; Strittmatter, W. J.; Schmechel, D. E.; Gaskell, P. C.; Small, G. W.; Roses, A. D.; Haines, J. L.; Pericak-Vance, M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993, 261, 921-3.
- Poirier, J.; Davignon, J.; Bouthillier, D.; Kogan, S.; Bertrand, P.; Gauthier, S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 1993, 342, 697-9.
- Rawat, V.; Wang, S.; Sima, J.; Bar, R.; Liraz, O.; Gundimeda, U.; Parekh, T.; Chan, J.; Johansson, J. O.; Tang, C.; Chui, H. C.; Harrington, M. G.; Michaelson, D. M.; Yassine, H. N. ApoE4 Alters ABCA1 membrane trafficking in astrocytes. J Neurosci 2019, 39, 9611-22.
- Marchi, C.; Adorni, M. P.; Caffarra, P.; Ronda, N.; Spallazzi, M.; Barocco, F.; Galimberti, D.; Bernini, F.; Zimetti, F. ABCA1- and ABCG1-mediated cholesterol efflux capacity of cerebrospinal fluid is impaired in Alzheimer's disease. J Lipid Res 2019, 60, 1449-56.
- Akram, A.; Schmeidler, J.; Katsel, P.; Hof, P. R.; Haroutunian, V. Increased expression of cholesterol transporter ABCA1 is highly correlated with severity of dementia in AD hippocampus. Brain Res 2010, 1318, 167-77.
- Holstege, H.; Hulsman, M.; Charbonnier, C.; Grenier-Boley, B.; Quenez, O.; Grozeva, D.; van Rooij, J. G. J.; Sims, R.; Ahmad, S.; Amin, N.; Norsworthy, P. J.; Dols-Icardo, O.; Hummerich, H.; Kawalia, A.; database, A. s. D. N. I.; Amouyel, P.; Beecham, G. W.; Berr, C.; Bis, J. C.; Boland, A.; Bossù, P.; Bouwman, F.; Bras, J.; Campion, D.; Cochran, J. N.; Daniele, A.; Dartigues, J.-F.; Debette, S.; Deleuze, J.-F.; Denning, N.; DeStefano, A. L.; Farrer, L. A.; Fernandez, M. V.; Fox, N. C.; Galimberti, D.; Genin, E.; Gille, H.; Guen, Y. L.; Guerreiro, R.; Haines, J. L.; Holmes, C.; Ikram, M. A.; Ikram, M. K.; Jansen, I. E.; Kraaij, R.; Lathrop, M.; Lemstra, A. W.; Lleó, A.; Luckcuck, L.; Mannens, M. M. A. M.; Marshall, R.; Martin, E. R.; Masullo, C.; Mayeux, R.; Mecocci, P.; Meggy, A.; Mol, M. O.; Morgan, K.; Myers, R. M.; Nacmias, B.; Naj, A. C.; Napolioni, V.; Pasquier, F.; Pastor, P.; Pericak-Vance, M. A.; Raybould, R.; Redon, R.; Reinders, M. J. T.; Richard, A.-C.; Riedel-Heller, S. G.; Rivadeneira, F.; Rousseau, S.; Ryan, N. S.; Saad, S.; Sanchez-Juan, P.; Schellenberg, G. D.; Scheltens, P.; Schott, J. M.; Seripa, D.; Seshadri, S.; Sie, D.; Sistermans, E.; Sorbi, S.; van Spaendonk, R.; Spalletta, G.; Tesi, N.; Tijms, B.; Uitterlinden, A. G.; van der Lee, S. J.; de Visser, P. J.; Wagner, M.; Wallon, D.; Wang, L.-S.; Zarea, A.; Clarimon, J.; van Swieten, J. C.; Greicius, M. D.; Yokoyama, J. S.; Cruchaga, C.; Hardy, J.; Ramirez, A.; Mead, S.; van der Flier, W. M.; van Duijn, C. M.; Williams, J.; Nicolas, G.; Bellenguez, C.; Lambert, J.-C. Exome sequencing identifies rare damaging variants in the ATP8B4 and ABCA1 genes as novel risk factors for Alzheimer’s Disease. medRxiv 2021, 2020.07.22.20159251.
- Alrasadi, K.; Ruel, I. L.; Marcil, M.; Genest, J. Functional mutations of the ABCA1 gene in subjects of French-Canadian descent with HDL deficiency. Atheroscler 2006, 188, 281-91.
- Ma, X.; Wang, T.; Zhao, Z. L.; Jiang, Y.; Ye, S. propofol suppresses proinflammatory cytokine production by increasing ABCA1 expression via mediation by the long noncoding RNA LOC286367. Mediators Inflamm 2018, 2018, 8907143.
- Koldamova, R.; Staufenbiel, M.; Lefterov, I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem 2005, 280, 43224-35.
- Wahrle, S. E.; Jiang, H.; Parsadanian, M.; Hartman, R. E.; Bales, K. R.; Paul, S. M.; Holtzman, D. M. Deletion of ABCA1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem 2005, 280, 43236-42.
- Hirsch-Reinshagen, V.; Maia, L. F.; Burgess, B. L.; Blain, J. F.; Naus, K. E.; McIsaac, S. A.; Parkinson, P. F.; Chan, J. Y.; Tansley, G. H.; Hayden, M. R.; Poirier, J.; Van Nostrand, W.; Wellington, C. L. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem 2005, 280, 43243-56.
- Fitz, N. F.; Nam, K. N.; Wolfe, C. M.; Letronne, F.; Playso, B. E.; Iordanova, B. E.; Kozai, T. D. Y.; Biedrzycki, R. J.; Kagan, V. E.; Tyurina, Y. Y.; Han, X.; Lefterov, I.; Koldamova, R. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer's disease. Nat Commun 2021, 12, 3416.
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M. B.; Holtzman, D. M.; Zlokovic, B. V. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008, 118, 4002-13.
- Basak, J. M.; Kim, J.; Pyatkivskyy, Y.; Wildsmith, K. R.; Jiang, H.; Parsadanian, M.; Patterson, B. W.; Bateman, R. J.; Holtzman, D. M. Measurement of apolipoprotein E and amyloid beta clearance rates in the mouse brain using bolus stable isotope labeling. Mol Neurodegener 2012, 7, 14.
- Fitz, N. F.; Tapias, V.; Cronican, A. A.; Castranio, E. L.; Saleem, M.; Carter, A. Y.; Lefterova, M.; Lefterov, I.; Koldamova, R. Opposing effects of Apoe/Apoa1 double deletion on amyloid-beta pathology and cognitive performance in APP mice. Brain 2015, 138, 3699-715.
- Lefterov, I.; Fitz, N. F.; Cronican, A.; Lefterov, P.; Staufenbiel, M.; Koldamova, R. Memory deficits in APP23/Abca1+/- mice correlate with the level of Abeta oligomers. ASN Neuro 2009, 1, e00006.
- Fitz, N. F.; Cronican, A. A.; Saleem, M.; Fauq, A. H.; Chapman, R.; Lefterov, I.; Koldamova, R. Abca1 deficiency affects Alzheimer's disease-like phenotype in human ApoE4 but not in ApoE3-targeted replacement mice. J Neurosci 2012, 32, 13125-36.
- Broccardo, C.; Nieoullon, V.; Amin, R.; Masmejean, F.; Carta, S.; Tassi, S.; Pophillat, M.; Rubartelli, A.; Pierres, M.; Rougon, G.; Nieoullon, A.; Chazal, G.; Chimini, G. ABCA2 is a marker of neural progenitors and neuronal subsets in the adult rodent brain. J Neurochem 2006, 97, 345-55.
- Vulevic, B.; Chen, Z.; Boyd, J. T.; Davis, W., Jr.; Walsh, E. S.; Belinsky, M. G.; Tew, K. D. Cloning and characterization of human adenosine 5'-triphosphate-binding cassette, sub-family A, transporter 2 (ABCA2). Cancer Res 2001, 61, 3339-47.
- Zhou, C.; Zhao, L.; Inagaki, N.; Guan, J.; Nakajo, S.; Hirabayashi, T.; Kikuyama, S.; Shioda, S. Atp-binding cassette transporter ABC2/ABCA2 in the rat brain: a novel mammalian lysosome-associated membrane protein and a specific marker for oligodendrocytes but not for myelin sheaths. J Neurosci 2001, 21, 849-57.
- Zhao, L. X.; Zhou, C. J.; Tanaka, A.; Nakata, M.; Hirabayashi, T.; Amachi, T.; Shioda, S.; Ueda, K.; Inagaki, N. Cloning, characterization and tissue distribution of the rat ATP-binding cassette (ABC) transporter ABC2/ABCA2. Biochem J 2000, 350 Pt 3, 865-72.
- Tanaka, Y.; Yamada, K.; Zhou, C. J.; Ban, N.; Shioda, S.; Inagaki, N. Temporal and spatial profiles of ABCA2-expressing oligodendrocytes in the developing rat brain. J Comp Neurol 2003, 455, 353-67.
- Kaminski, W. E.; Piehler, A.; Püllmann, K.; Porsch-Ozcürümez, M.; Duong, C.; Bared, G. M.; Büchler, C.; Schmitz, G. Complete coding sequence, promoter region, and genomic structure of the human ABCA2 gene and evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun 2001, 281, 249-58.
- Wollmer, M. A.; Kapaki, E.; Hersberger, M.; Muntwyler, J.; Brunner, F.; Tsolaki, M.; Akatsu, H.; Kosaka, K.; Michikawa, M.; Molyva, D.; Paraskevas, G. P.; Lütjohann, D.; von Eckardstein, A.; Hock, C.; Nitsch, R. M.; Papassotiropoulos, A. Ethnicity-dependent genetic association of ABCA2 with sporadic Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet 2006, 141b, 534-6.
- Minster, R. L.; DeKosky, S. T.; Kamboh, M. I. No association of DAPK1 and ABCA2 SNPs on chromosome 9 with Alzheimer's disease. Neurobiol Aging 2009, 30, 1890-1.
- Hu, W.; Lin, X.; Zhang, H.; Zhao, N. ATP binding cassette subfamily a member 2 (ABCA2) expression and methylation are associated with alzheimer's disease. Med Sci Monit 2017, 23, 5851-61.
- Davis, W., Jr.; Boyd, J. T.; Ile, K. E.; Tew, K. D. Human ATP-binding cassette transporter-2 (ABCA2) positively regulates low-density lipoprotein receptor expression and negatively regulates cholesterol esterification in Chinese hamster ovary cells. Biochim Biophys Acta 2004, 1683, 89-100.
- Chen, Z. J.; Vulevic, B.; Ile, K. E.; Soulika, A.; Davis, W., Jr.; Reiner, P. B.; Connop, B. P.; Nathwani, P.; Trojanowski, J. Q.; Tew, K. D. Association of ABCA2 expression with determinants of Alzheimer's disease. FASEB J 2004, 18, 1129-31.
- Katsouri, L.; Georgopoulos, S. Lack of LDL receptor enhances amyloid deposition and decreases glial response in an Alzheimer's disease mouse model. PloS one 2011, 6, e21880.
- Michaki, V.; Guix, F. X.; Vennekens, K.; Munck, S.; Dingwall, C.; Davis, J. B.; Townsend, D. M.; Tew, K. D.; Feiguin, F.; De Strooper, B.; Dotti, C. G.; Wahle, T. Down-regulation of the ATP-binding cassette transporter 2 (Abca2) reduces amyloid-beta production by altering Nicastrin maturation and intracellular localization. J Biol Chem 2012, 287, 1100-11.
- Davis, W., Jr. The ATP-binding cassette transporter-2 (ABCA2) overexpression modulates sphingosine levels and transcription of the amyloid precursor protein (APP) gene. Curr Alzheimer Res 2015, 12, 847-59.
- Kim, W. S.; Rahmanto, A. S.; Kamili, A.; Rye, K. A.; Guillemin, G. J.; Gelissen, I. C.; Jessup, W.; Hill, A. F.; Garner, B. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. J Biol Chem 2007, 282, 2851-61.
- Yamano, G.; Funahashi, H.; Kawanami, O.; Zhao, L. X.; Ban, N.; Uchida, Y.; Morohoshi, T.; Ogawa, J.; Shioda, S.; Inagaki, N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett 2001, 508, 221-5.
- Fitzgerald, M. L.; Xavier, R.; Haley, K. J.; Welti, R.; Goss, J. L.; Brown, C. E.; Zhuang, D. Z.; Bell, S. A.; Lu, N.; McKee, M.; Seed, B.; Freeman, M. W. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res 2007, 48, 621-32.
- Stahlman, M. T.; Besnard, V.; Wert, S. E.; Weaver, T. E.; Dingle, S.; Xu, Y.; von Zychlin, K.; Olson, S. J.; Whitsett, J. A. Expression of ABCA3 in developing lung and other tissues. J Histochem Cytochem 2007, 55, 71-83.
- Kim, W. S.; Guillemin, G. J.; Glaros, E. N.; Lim, C. K.; Garner, B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport 2006, 17, 891-6.
- Whiley, L.; Sen, A.; Heaton, J.; Proitsi, P.; García-Gómez, D.; Leung, R.; Smith, N.; Thambisetty, M.; Kloszewska, I.; Mecocci, P.; Soininen, H.; Tsolaki, M.; Vellas, B.; Lovestone, S.; Legido-Quigley, C. Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging 2014, 35, 271-8.
- Allikmets, R.; Singh, N.; Sun, H.; Shroyer, N. F.; Hutchinson, A.; Chidambaram, A.; Gerrard, B.; Baird, L.; Stauffer, D.; Peiffer, A.; Rattner, A.; Smallwood, P.; Li, Y.; Anderson, K. L.; Lewis, R. A.; Nathans, J.; Leppert, M.; Dean, M.; Lupski, J. R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 1997, 15, 236-46.
- Kaway, C. S.; Adams, M. K. M.; Jenkins, K. S.; Layton, C. J. A Novel ABCA4 mutation associated with a late-onset stargardt disease phenotype: A hypomorphic allele? Case Rep Ophthalmol 2017, 8, 180-4.
- Muller, P. L.; Treis, T.; Odainic, A.; Pfau, M.; Herrmann, P.; Tufail, A.; Holz, F. G. Prediction of function in ABCA4-related retinopathy using ensemble machine learning. J Clin Med 2020, 9.
- Ohtsuki, S.; Watanabe, Y.; Hori, S.; Suzuki, H.; Bhongsatiern, J.; Fujiyoshi, M.; Kamoi, M.; Kamiya, N.; Takanaga, H.; Terasaki, T. mRNA expression of the ATP-binding cassette transporter subfamily A (ABCA) in rat and human brain capillary endothelial cells. Biol Pharm Bull 2004, 27, 1437-40.
- Petry, F.; Kotthaus, A.; Hirsch-Ernst, K. I. Cloning of human and rat ABCA5/Abca5 and detection of a human splice variant. Biochem Biophys Res Commun 2003, 300, 343-50.
- Fu, Y.; Hsiao, J. H.; Paxinos, G.; Halliday, G. M.; Kim, W. S. ABCA5 regulates amyloid-beta peptide production and is associated with Alzheimer's disease neuropathology. J Alzheimers Dis 2015, 43, 857-69.
- Kaminski, W. E.; Wenzel, J. J.; Piehler, A.; Langmann, T.; Schmitz, G. ABCA6, a novel a subclass ABC transporter. Biochem Biophys Res Commun 2001, 285, 1295-301.
- van Leeuwen, E. M.; Karssen, L. C.; Deelen, J.; Isaacs, A.; Medina-Gomez, C.; Mbarek, H.; Kanterakis, A.; Trompet, S.; Postmus, I.; Verweij, N.; van Enckevort, D. J.; Huffman, J. E.; White, C. C.; Feitosa, M. F.; Bartz, T. M.; Manichaikul, A.; Joshi, P. K.; Peloso, G. M.; Deelen, P.; van Dijk, F.; Willemsen, G.; de Geus, E. J.; Milaneschi, Y.; Penninx, B. W.; Francioli, L. C.; Menelaou, A.; Pulit, S. L.; Rivadeneira, F.; Hofman, A.; Oostra, B. A.; Franco, O. H.; Mateo Leach, I.; Beekman, M.; de Craen, A. J.; Uh, H. W.; Trochet, H.; Hocking, L. J.; Porteous, D. J.; Sattar, N.; Packard, C. J.; Buckley, B. M.; Brody, J. A.; Bis, J. C.; Rotter, J. I.; Mychaleckyj, J. C.; Campbell, H.; Duan, Q.; Lange, L. A.; Wilson, J. F.; Hayward, C.; Polasek, O.; Vitart, V.; Rudan, I.; Wright, A. F.; Rich, S. S.; Psaty, B. M.; Borecki, I. B.; Kearney, P. M.; Stott, D. J.; Adrienne Cupples, L.; Jukema, J. W.; van der Harst, P.; Sijbrands, E. J.; Hottenga, J. J.; Uitterlinden, A. G.; Swertz, M. A.; van Ommen, G. J.; de Bakker, P. I.; Eline Slagboom, P.; Boomsma, D. I.; Wijmenga, C.; van Duijn, C. M. Genome of The Netherlands population-specific imputations identify an ABCA6 variant associated with cholesterol levels. Nat Commun 2015, 6, 6065.
- Dib, S.; Pahnke, J.; Gosselet, F. Role of ABCA7 in human health and in Alzheimer's disease. Int J Mol Sci 2021, 22, 4603.
- Kaminski, W. E.; Piehler, A.; Schmitz, G. Genomic organization of the human cholesterol-responsive ABC transporter ABCA7: tandem linkage with the minor histocompatibility antigen HA-1 gene. Biochem Biophys Res Commun 2000, 278, 782-9.
- Kaminski, W. E.; Orso, E.; Diederich, W.; Klucken, J.; Drobnik, W.; Schmitz, G. Identification of a novel human sterol-sensitive ATP-binding cassette transporter (ABCA7). Biochem Biophys Res Commun 2000, 273, 532-8.
- Quazi, F.; Molday, R. S. Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J Biol Chem 2013, 288, 34414-26.
- Hayashi, M.; Abe-Dohmae, S.; Okazaki, M.; Ueda, K.; Yokoyama, S. Heterogeneity of high density lipoprotein generated by ABCA1 and ABCA7. J Lipid Res 2005, 46, 1703-11.
- Tanaka, N.; Abe-Dohmae, S.; Iwamoto, N.; Yokoyama, S. Roles of ATP-binding cassette transporter A7 in cholesterol homeostasis and host defense system. J Atheroscler Thromb 2011, 18, 274-81.
- Jehle, A. W.; Gardai, S. J.; Li, S.; Linsel-Nitschke, P.; Morimoto, K.; Janssen, W. J.; Vandivier, R. W.; Wang, N.; Greenberg, S.; Dale, B. M.; Qin, C.; Henson, P. M.; Tall, A. R. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol 2006, 174, 547-56.
- Iwamoto, N.; Abe-Dohmae, S.; Sato, R.; Yokoyama, S. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J Lipid Res 2006, 47, 1915-27.
- Meurs, I.; Calpe-Berdiel, L.; Habets, K. L.; Zhao, Y.; Korporaal, S. J.; Mommaas, A. M.; Josselin, E.; Hildebrand, R. B.; Ye, D.; Out, R.; Kuiper, J.; Van Berkel, T. J.; Chimini, G.; Van Eck, M. Effects of deletion of macrophage ABCA7 on lipid metabolism and the development of atherosclerosis in the presence and absence of ABCA1. PloS one 2012, 7, e30984.
- Kim, W. S.; Fitzgerald, M. L.; Kang, K.; Okuhira, K.; Bell, S. A.; Manning, J. J.; Koehn, S. L.; Lu, N.; Moore, K. J.; Freeman, M. W. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem 2005, 280, 3989-95.
- Linsel-Nitschke, P.; Jehle, A. W.; Shan, J.; Cao, G.; Bacic, D.; Lan, D.; Wang, N.; Tall, A. R. Potential role of ABCA7 in cellular lipid efflux to apoA-I. J Lipid Res 2005, 46, 86-92.
- Hollingworth, P.; Harold, D.; Sims, R.; Gerrish, A.; Lambert, J. C.; Carrasquillo, M. M.; Abraham, R.; Hamshere, M. L.; Pahwa, J. S.; Moskvina, V.; Dowzell, K.; Jones, N.; Stretton, A.; Thomas, C.; Richards, A.; Ivanov, D.; Widdowson, C.; Chapman, J.; Lovestone, S.; Powell, J.; Proitsi, P.; Lupton, M. K.; Brayne, C.; Rubinsztein, D. C.; Gill, M.; Lawlor, B.; Lynch, A.; Brown, K. S.; Passmore, P. A.; Craig, D.; McGuinness, B.; Todd, S.; Holmes, C.; Mann, D.; Smith, A. D.; Beaumont, H.; Warden, D.; Wilcock, G.; Love, S.; Kehoe, P. G.; Hooper, N. M.; Vardy, E. R.; Hardy, J.; Mead, S.; Fox, N. C.; Rossor, M.; Collinge, J.; Maier, W.; Jessen, F.; Ruther, E.; Schurmann, B.; Heun, R.; Kolsch, H.; van den Bussche, H.; Heuser, I.; Kornhuber, J.; Wiltfang, J.; Dichgans, M.; Frolich, L.; Hampel, H.; Gallacher, J.; Hull, M.; Rujescu, D.; Giegling, I.; Goate, A. M.; Kauwe, J. S.; Cruchaga, C.; Nowotny, P.; Morris, J. C.; Mayo, K.; Sleegers, K.; Bettens, K.; Engelborghs, S.; De Deyn, P. P.; Van Broeckhoven, C.; Livingston, G.; Bass, N. J.; Gurling, H.; McQuillin, A.; Gwilliam, R.; Deloukas, P.; Al-Chalabi, A.; Shaw, C. E.; Tsolaki, M.; Singleton, A. B.; Guerreiro, R.; Muhleisen, T. W.; Nothen, M. M.; Moebus, S.; Jockel, K. H.; Klopp, N.; Wichmann, H. E.; Pankratz, V. S.; Sando, S. B.; Aasly, J. O.; Barcikowska, M.; Wszolek, Z. K.; Dickson, D. W.; Graff-Radford, N. R.; Petersen, R. C.; Alzheimer's Disease Neuroimaging, I.; van Duijn, C. M.; Breteler, M. M.; Ikram, M. A.; DeStefano, A. L.; Fitzpatrick, A. L.; Lopez, O.; Launer, L. J.; Seshadri, S.; consortium, C.; Berr, C.; Campion, D.; Epelbaum, J.; Dartigues, J. F.; Tzourio, C.; Alperovitch, A.; Lathrop, M.; consortium, E.; Feulner, T. M.; Friedrich, P.; Riehle, C.; Krawczak, M.; Schreiber, S.; Mayhaus, M.; Nicolhaus, S.; Wagenpfeil, S.; Steinberg, S.; Stefansson, H.; Stefansson, K.; Snaedal, J.; Bjornsson, S.; Jonsson, P. V.; Chouraki, V.; Genier-Boley, B.; Hiltunen, M.; Soininen, H.; Combarros, O.; Zelenika, D.; Delepine, M.; Bullido, M. J.; Pasquier, F.; Mateo, I.; Frank-Garcia, A.; Porcellini, E.; Hanon, O.; Coto, E.; Alvarez, V.; Bosco, P.; Siciliano, G.; Mancuso, M.; Panza, F.; Solfrizzi, V.; Nacmias, B.; Sorbi, S.; Bossu, P.; Piccardi, P.; Arosio, B.; Annoni, G.; Seripa, D.; Pilotto, A.; Scarpini, E.; Galimberti, D.; Brice, A.; Hannequin, D.; Licastro, F.; Jones, L.; Holmans, P. A.; Jonsson, T.; Riemenschneider, M.; Morgan, K.; Younkin, S. G.; Owen, M. J.; O'Donovan, M.; Amouyel, P.; Williams, J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet 2011, 43, 429-35.
- Vasquez, J. B.; Simpson, J. F.; Harpole, R.; Estus, S. Alzheimer's disease genetics and ABCA7 splicing. J Alzheimers Dis 2017, 59, 633-41.
- Karch, C. M.; Jeng, A. T.; Nowotny, P.; Cady, J.; Cruchaga, C.; Goate, A. M. Expression of novel Alzheimer's disease risk genes in control and Alzheimer's disease brains. PloS one 2012, 7, e50976.
- Allen, M.; Zou, F.; Chai, H. S.; Younkin, C. S.; Crook, J.; Pankratz, V. S.; Carrasquillo, M. M.; Rowley, C. N.; Nair, A. A.; Middha, S.; Maharjan, S.; Nguyen, T.; Ma, L.; Malphrus, K. G.; Palusak, R.; Lincoln, S.; Bisceglio, G.; Georgescu, C.; Schultz, D.; Rakhshan, F.; Kolbert, C. P.; Jen, J.; Haines, J. L.; Mayeux, R.; Pericak-Vance, M. A.; Farrer, L. A.; Schellenberg, G. D.; Petersen, R. C.; Graff-Radford, N. R.; Dickson, D. W.; Younkin, S. G.; Ertekin-Taner, N.; Alzheimer's Disease Genetics, C.; Apostolova, L. G.; Arnold, S. E.; Baldwin, C. T.; Barber, R.; Barmada, M. M.; Beach, T.; Beecham, G. W.; Beekly, D.; Bennett, D. A.; Bigio, E. H.; Bird, T. D.; Blacker, D.; Boeve, B. F.; Bowen, J. D.; Boxer, A.; Burke, J. R.; Buros, J.; Buxbaum, J. D.; Cairns, N. J.; Cantwell, L. B.; Cao, C.; Carlson, C. S.; Carney, R. M.; Carroll, S. L.; Chui, H. C.; Clark, D. G.; Corneveaux, J.; Cotman, C. W.; Crane, P. K.; Cruchaga, C.; Cummings, J. L.; De Jager, P. L.; DeCarli, C.; DeKosky, S. T.; Demirci, F. Y.; Diaz-Arrastia, R.; Dick, M.; Dombroski, B. A.; Duara, R.; Ellis, W. D.; Evans, D.; Faber, K. M.; Fallon, K. B.; Farlow, M. R.; Ferris, S.; Foroud, T. M.; Frosch, M.; Galasko, D. R.; Gallins, P. J.; Ganguli, M.; Gearing, M.; Geschwind, D. H.; Ghetti, B.; Gilbert, J. R.; Gilman, S.; Giordani, B.; Glass, J. D.; Goate, A. M.; Green, R. C.; Growdon, J. H.; Hakonarson, H.; Hamilton, R. L.; Hardy, J.; Harrell, L. E.; Head, E.; Honig, L. S.; Huentelman, M. J.; Hulette, C. M.; Hyman, B. T.; Jarvik, G. P.; Jicha, G. A.; Jin, L. W.; Jun, G.; Kamboh, M. I.; Karlawish, J.; Karydas, A.; Kauwe, J. S.; Kaye, J. A.; Kennedy, N.; Kim, R.; Koo, E. H.; Kowall, N. W.; Kramer, P.; Kukull, W. A.; Lah, J. J.; Larson, E. B.; Levey, A. I.; Lieberman, A. P.; Lopez, O. L.; Lunetta, K. L.; Mack, W. J.; Marson, D. C.; Martin, E. R.; Martiniuk, F.; Mash, D. C.; Masliah, E.; McCormick, W. C.; McCurry, S. M.; McDavid, A. N.; McKee, A. C.; Mesulam, M.; Miller, B. L.; Miller, C. A.; Miller, J. W.; Montine, T. J.; Morris, J. C.; Myers, A. J.; Naj, A. C.; Nowotny, P.; Parisi, J. E.; Perl, D. P.; Peskind, E.; Poon, W. W.; Potter, H.; Quinn, J. F.; Raj, A.; Rajbhandary, R. A.; Raskind, M.; Reiman, E. M.; Reisberg, B.; Reitz, C.; Ringman, J. M.; Roberson, E. D.; Rogaeva, E.; Rosenberg, R. N.; Sano, M.; Saykin, A. J.; Schneider, J. A.; Schneider, L. S.; Seeley, W.; Shelanski, M. L.; Slifer, M. A.; Smith, C. D.; Sonnen, J. A.; Spina, S.; St George-Hyslop, P.; Stern, R. A.; Tanzi, R. E.; Trojanowski, J. Q.; Troncoso, J. C.; Tsuang, D. W.; Van Deerlin, V. M.; Vardarajan, B. N.; Vinters, H. V.; Vonsattel, J. P.; Wang, L. S.; Weintraub, S.; Welsh-Bohmer, K. A.; Williamson, J.; Woltjer, R. L. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurol 2012, 79, 221-8.
- Steinberg, S.; Stefansson, H.; Jonsson, T.; Johannsdottir, H.; Ingason, A.; Helgason, H.; Sulem, P.; Magnusson, O. T.; Gudjonsson, S. A.; Unnsteinsdottir, U.; Kong, A.; Helisalmi, S.; Soininen, H.; Lah, J. J.; DemGene; Aarsland, D.; Fladby, T.; Ulstein, I. D.; Djurovic, S.; Sando, S. B.; White, L. R.; Knudsen, G. P.; Westlye, L. T.; Selbaek, G.; Giegling, I.; Hampel, H.; Hiltunen, M.; Levey, A. I.; Andreassen, O. A.; Rujescu, D.; Jonsson, P. V.; Bjornsson, S.; Snaedal, J.; Stefansson, K. Loss-of-function variants in ABCA7 confer risk of Alzheimer's disease. Nat Genet 2015, 47, 445-7.
- Shulman, J. M.; Chen, K.; Keenan, B. T.; Chibnik, L. B.; Fleisher, A.; Thiyyagura, P.; Roontiva, A.; McCabe, C.; Patsopoulos, N. A.; Corneveaux, J. J.; Yu, L.; Huentelman, M. J.; Evans, D. A.; Schneider, J. A.; Reiman, E. M.; De Jager, P. L.; Bennett, D. A. Genetic susceptibility for Alzheimer disease neuritic plaque pathology. JAMA Neurol 2013, 70, 1150-7.
- Sinha, N.; Berg, C. N.; Shaw, A.; Gluck, M. A. ABCA7 genotype moderates the effect of aerobic exercise intervention on generalization of prior learning in healthy older African Americans. J Alzheimers Dis 2020, 74, 309-18.
- Lamartiniere, Y.; Boucau, M. C.; Dehouck, L.; Krohn, M.; Pahnke, J.; Candela, P.; Gosselet, F.; Fenart, L. ABCA7 downregulation modifies cellular cholesterol homeostasis and decreases amyloid-beta peptide efflux in an in vitro model of the blood-brain barrier. J Alzheimers Dis 2018, 64, 1195-1211.
- Li, M.; Yuan, Y.; Hu, B.; Wu, L. Study on lentivirus-mediated ABCA7 improves neurocognitive function and related mechanisms in the C57BL/6 mouse model of alzheimer's disease. J Mol Neurosci 2017, 61, 489-97.
- Sakae, N.; Liu, C. C.; Shinohara, M.; Frisch-Daiello, J.; Ma, L.; Yamazaki, Y.; Tachibana, M.; Younkin, L.; Kurti, A.; Carrasquillo, M. M.; Zou, F.; Sevlever, D.; Bisceglio, G.; Gan, M.; Fol, R.; Knight, P.; Wang, M.; Han, X.; Fryer, J. D.; Fitzgerald, M. L.; Ohyagi, Y.; Younkin, S. G.; Bu, G.; Kanekiyo, T. ABCA7 deficiency accelerates amyloid-beta generation and Alzheimer's neuronal pathology. J Neurosci 2016, 36, 3848-59.
- Aikawa, T.; Ren, Y.; Yamazaki, Y.; Tachibana, M.; Johnson, M. R.; Anderson, C. T.; Martens, Y. A.; Holm, M. L.; Asmann, Y. W.; Saito, T.; Saido, T. C.; Fitzgerald, M. L.; Bu, G.; Kanekiyo, T. ABCA7 haplodeficiency disturbs microglial immune responses in the mouse brain. Proc Natl Acad Sci U S A 2019, 116, 23790-23796.
- Zissimopoulos, J. M.; Barthold, D.; Brinton, R. D.; Joyce, G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurolog 2017, 74, 225-32.
- Yang, C.; Yuan, H.; Gu, J.; Xu, D.; Wang, M.; Qiao, J.; Yang, X.; Zhang, J.; Yao, M.; Gu, J.; Tu, H.; Gan, Y. ABCA8-mediated efflux of taurocholic acid contributes to gemcitabine insensitivity in human pancreatic cancer via the S1PR2-ERK pathway. Cell Death Discov 2021, 7, 6.
- Tsuruoka, S.; Ishibashi, K.; Yamamoto, H.; Wakaumi, M.; Suzuki, M.; Schwartz, G. J.; Imai, M.; Fujimura, A. Functional analysis of ABCA8, a new drug transporter. Biochem Biophys Res Commun 2002, 298, 41-5.
- Akiyama, M.; Sugiyama-Nakagiri, Y.; Sakai, K.; McMillan, J. R.; Goto, M.; Arita, K.; Tsuji-Abe, Y.; Tabata, N.; Matsuoka, K.; Sasaki, R.; Sawamura, D.; Shimizu, H. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest 2005, 115, 1777-84.
- Kelsell, D. P.; Norgett, E. E.; Unsworth, H.; Teh, M. T.; Cullup, T.; Mein, C. A.; Dopping-Hepenstal, P. J.; Dale, B. A.; Tadini, G.; Fleckman, P.; Stephens, K. G.; Sybert, V. P.; Mallory, S. B.; North, B. V.; Witt, D. R.; Sprecher, E.; Taylor, A. E.; Ilchyshyn, A.; Kennedy, C. T.; Goodyear, H.; Moss, C.; Paige, D.; Harper, J. I.; Young, B. D.; Leigh, I. M.; Eady, R. A.; O'Toole, E. A. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet 2005, 76, 794-803.
- Lefévre, C.; Audebert, S.; Jobard, F.; Bouadjar, B.; Lakhdar, H.; Boughdene-Stambouli, O.; Blanchet-Bardon, C.; Heilig, R.; Foglio, M.; Weissenbach, J.; Lathrop, M.; Prud'homme, J. F.; Fischer, J. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet 2003, 12, 2369-78.
- Ohkubo, T.; Shibata, N.; Ohnuma, T.; Higashi, S.; Usui, C.; Ueki, A.; Nagao, M.; Arai, H. No genetic association between ATP binding cassette proteins and Japanese sporadic Alzheimer's disease. Dement Geriatr Cogn Disord 2005, 20, 95-8.
- Prades, C.; Arnould, I.; Annilo, T.; Shulenin, S.; Chen, Z. Q.; Orosco, L.; Triunfol, M.; Devaud, C.; Maintoux-Larois, C.; Lafargue, C.; Lemoine, C.; Denèfle, P.; Rosier, M.; Dean, M. The human ATP binding cassette gene ABCA13, located on chromosome 7p12.3, encodes a 5058 amino acid protein with an extracellular domain encoded in part by a 4.8-kb conserved exon. Cytogenet Genome Res 2002, 98, 160-8.
- Kim, W. S.; Weickert, C. S.; Garner, B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem 2008, 104, 1145-66.
- Sato, K.; Malchinkhuu, E.; Horiuchi, Y.; Mogi, C.; Tomura, H.; Tosaka, M.; Yoshimoto, Y.; Kuwabara, A.; Okajima, F. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem 2007, 103, 2610-9.
- Zhao, X.; Murata, T.; Ohno, S.; Day, N.; Song, J.; Nomura, N.; Nakahara, T.; Yokoyama, K. K. Protein kinase Calpha plays a critical role in mannosylerythritol lipid-induced differentiation of melanoma B16 cells. J Biol Chem 2001, 276, 39903-10.
- Olivier, M.; Bott, G. R.; Frisdal, E.; Nowick, M.; Plengpanich, W.; Desmarchelier, C.; Roi, S.; Quinn, C. M.; Gelissen, I.; Jessup, W.; Van Eck, M.; Guerin, M.; Le Goff, W.; Reboul, E. ABCG1 is involved in vitamin E efflux. Biochim Biophys Acta 2014, 1841, 1741-51.
- Stefan, S. M.; Jansson, P. J.; Kalinowski, D. S.; Anjum, R.; Dharmasivam, M.; Richardson, D. R. The growing evidence for targeting P-glycoprotein in lysosomes to overcome resistance. Future Med Chem 2020, 12, 473-477.
- Zhitomirsky, B.; Assaraf, Y. G. Lysosomes as mediators of drug resistance in cancer. Drug Resist Updat 2016, 24, 23-33.
- Chapuy, B.; Panse, M.; Radunski, U.; Koch, R.; Wenzel, D.; Inagaki, N.; Haase, D.; Truemper, L.; Wulf, G. G. ABC transporter A3 facilitates lysosomal sequestration of imatinib and modulates susceptibility of chronic myeloid leukemia cell lines to this drug. Haematol 2009, 94, 1528-36.
- Chapuy, B.; Koch, R.; Radunski, U.; Corsham, S.; Cheong, N.; Inagaki, N.; Ban, N.; Wenzel, D.; Reinhardt, D.; Zapf, A.; Schweyer, S.; Kosari, F.; Klapper, W.; Truemper, L.; Wulf, G. G. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leuk 2008, 22, 1576-86.
- Dharmapuri, G.; Doneti, R.; Philip, G. H.; Kalle, A. M. Celecoxib sensitizes imatinib-resistant K562 cells to imatinib by inhibiting MRP1-5, ABCA2 and ABCG2 transporters via Wnt and Ras signaling pathways. Leuk Res 2015, 39, 696-701.
- Steinbach, D.; Gillet, J. P.; Sauerbrey, A.; Gruhn, B.; Dawczynski, K.; Bertholet, V.; de Longueville, F.; Zintl, F.; Remacle, J.; Efferth, T. ABCA3 as a possible cause of drug resistance in childhood acute myeloid leukemia. Clin Cancer Res 2006, 12, 4357-63.
- Laing, N. M.; Belinsky, M. G.; Kruh, G. D.; Bell, D. W.; Boyd, J. T.; Barone, L.; Testa, J. R.; Tew, K. D. Amplification of the ATP-binding cassette 2 transporter gene is functionally linked with enhanced efflux of estramustine in ovarian carcinoma cells. Cancer Res 1998, 58, 1332-7.
- Mack, J. T.; Brown, C. B.; Garrett, T. E.; Uys, J. D.; Townsend, D. M.; Tew, K. D. Ablation of the ATP-binding cassette transporter, Abca2 modifies response to estrogen-based therapies. Biomed Pharmacother 2012, 66, 403-8.
- Dohmen, L. C.; Navas, A.; Vargas, D. A.; Gregory, D. J.; Kip, A.; Dorlo, T. P.; Gomez, M. A. functional validation of ABCA3 as a miltefosine transporter in human macrophages: Impact on intracellular survival of leishmania (viannia) panamensis. J Biol Chem 2016, 291, 9638-47.
- Overbeck, T. R.; Hupfeld, T.; Krause, D.; Waldmann-Beushausen, R.; Chapuy, B.; Guldenzoph, B.; Aung, T.; Inagaki, N.; Schondube, F. A.; Danner, B. C.; Truemper, L.; Wulf, G. G. Intracellular ATP-binding cassette transporter A3 is expressed in lung cancer cells and modulates susceptibility to cisplatin and paclitaxel. Oncol 2013, 84, 362-70.
- Aberuyi, N.; Rahgozar, S.; Pourabutaleb, E.; Ghaedi, K. Selective dysregulation of ABC transporters in methotrexate-resistant leukemia T-cells can confer cross-resistance to cytarabine, vincristine and dexamethasone, but not doxorubicin. Curr Res Transl Med 2021, 69, 103269.
- Shroyer, N. F.; Lewis, R. A.; Lupski, J. R. Analysis of the ABCR (ABCA4) gene in 4-aminoquinoline retinopathy: is retinal toxicity by chloroquine and hydroxychloroquine related to Stargardt disease? Am J Ophthalmol 2001, 131, 761-6.
- Mack, H. G.; Kowalski, T.; Lucattini, A.; Symons, R. A.; Wicks, I.; Hall, A. J. Genetic susceptibility to hydroxychloroquine retinal toxicity. Ophthalmic Genet 2020, 41, 159-70.
- Nagao, K.; Maeda, M.; Manucat, N. B.; Ueda, K. Cyclosporine A and PSC833 inhibit ABCA1 function via direct binding. Biochim Biophys Acta 2013, 1831, 398-406.
- Wu, C. A.; Tsujita, M.; Hayashi, M.; Yokoyama, S. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem 2004, 279, 30168-74.
- Hamon, Y.; Luciani, M. F.; Becq, F.; Verrier, B.; Rubartelli, A.; Chimini, G. Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 1997, 90, 2911-5.
- Becq, F.; Hamon, Y.; Bajetto, A.; Gola, M.; Verrier, B.; Chimini, G. ABC1, an ATP binding cassette transporter required for phagocytosis of apoptotic cells, generates a regulated anion flux after expression in Xenopus laevis oocytes. J Biol Chem 1997, 272, 2695-9.
- Le Goff, W.; Peng, D. Q.; Settle, M.; Brubaker, G.; Morton, R. E.; Smith, J. D. Cyclosporin A traps ABCA1 at the plasma membrane and inhibits ABCA1-mediated lipid efflux to apolipoprotein A-I. Arterioscler Thromb Vasc Biol 2004, 24, 2155-61.
- Monzel, J. V.; Budde, T.; Meyer Zu Schwabedissen, H. E.; Schwebe, M.; Bien-Moller, S.; Lutjohann, D.; Kroemer, H. K.; Jedlitschky, G.; Grube, M. Doxorubicin enhances oxysterol levels resulting in a LXR-mediated upregulation of cardiac cholesterol transporters. Biochem Pharmacol 2017, 144, 108-119.
- Burns, V. E.; Kerppola, T. K. ATR-101 inhibits cholesterol efflux and cortisol secretion by ATP-binding cassette transporters, causing cytotoxic cholesterol accumulation in adrenocortical carcinoma cells. Br J Pharmacol 2017, 174, 3315-32.
- Zhao, J. F.; Jim Leu, S. J.; Shyue, S. K.; Su, K. H.; Wei, J.; Lee, T. S. Novel effect of paeonol on the formation of foam cells: promotion of LXRalpha-ABCA1-dependent cholesterol efflux in macrophages. Am J Chin Med 2013, 41, 1079-96.
- Campia, I.; Sala, V.; Kopecka, J.; Leo, C.; Mitro, N.; Costamagna, C.; Caruso, D.; Pescarmona, G.; Crepaldi, T.; Ghigo, D.; Bosia, A.; Riganti, C. Digoxin and ouabain induce the efflux of cholesterol via liver X receptor signalling and the synthesis of ATP in cardiomyocytes. Biochem J 2012, 447, 301-11.
- Chen, C. Y.; Shyue, S. K.; Ching, L. C.; Su, K. H.; Wu, Y. L.; Kou, Y. R.; Chiang, A. N.; Pan, C. C.; Lee, T. S. Wogonin promotes cholesterol efflux by increasing protein phosphatase 2B-dependent dephosphorylation at ATP-binding cassette transporter-A1 in macrophages. J Nutr Biochem 2011, 22, 1015-21.
- Howard, A. D.; Verghese, P. B.; Arrese, E. L.; Soulages, J. L. Characterization of apoA-I-dependent lipid efflux from adipocytes and role of ABCA1. Mol Cell Biochem 2010, 343, 115-24.
- Lee, T. S.; Pan, C. C.; Peng, C. C.; Kou, Y. R.; Chen, C. Y.; Ching, L. C.; Tsai, T. H.; Chen, S. F.; Lyu, P. C.; Shyue, S. K. Anti-atherogenic effect of berberine on LXRalpha-ABCA1-dependent cholesterol efflux in macrophages. J Cell Biochem 2010, 111, 104-10.
- Duncan, K. G.; Hosseini, K.; Bailey, K. R.; Yang, H.; Lowe, R. J.; Matthes, M. T.; Kane, J. P.; LaVail, M. M.; Schwartz, D. M.; Duncan, J. L. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br J Ophthalmol 2009, 93, 1116-20.
- Wang, J.; Zhang, Z. R.; Chou, C. F.; Liang, Y. Y.; Gu, Y.; Ma, H. P. Cyclosporine stimulates the renal epithelial sodium channel by elevating cholesterol. Am J Physiol Renal Physiol 2009, 296, F284-90.
- Pirillo, A.; Uboldi, P.; Pappalardo, G.; Kuhn, H.; Catapano, A. L. Modification of HDL3 by mild oxidative stress increases ATP-binding cassette transporter 1-mediated cholesterol efflux. Cardiovasc Res 2007, 75, 566-74.
- Nofer, J. R.; Remaley, A. T.; Feuerborn, R.; Wolinnska, I.; Engel, T.; von Eckardstein, A.; Assmann, G. Apolipoprotein A-I activates Cdc42 signaling through the ABCA1 transporter. J Lipid Res 2006, 47, 794-803.
- Alder-Baerens, N.; Muller, P.; Pohl, A.; Korte, T.; Hamon, Y.; Chimini, G.; Pomorski, T.; Herrmann, A. Headgroup-specific exposure of phospholipids in ABCA1-expressing cells. J Biol Chem 2005, 280, 26321-9.
- Field, F. J.; Born, E.; Mathur, S. N. LXR/RXR ligand activation enhances basolateral efflux of beta-sitosterol in CaCo-2 cells. J Lipid Res 2004, 45, 905-13.
- Reddy, S. T.; Hama, S.; Ng, C.; Grijalva, V.; Navab, M.; Fogelman, A. M. ATP-binding cassette transporter 1 participates in LDL oxidation by artery wall cells. Arterioscler Thromb Vasc Biol 2002, 22, 1877-83.
- Murthy, S.; Born, E.; Mathur, S. N.; Field, F. J. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J Lipid Res 2002, 43, 1054-64.
- Huang, Z. H.; Lin, C. Y.; Oram, J. F.; Mazzone, T. Sterol efflux mediated by endogenous macrophage ApoE expression is independent of ABCA1. Arterioscler Thromb Vasc Biol 2001, 21, 2019-25.
- Von Eckardstein, A.; Langer, C.; Engel, T.; Schaukal, I.; Cignarella, A.; Reinhardt, J.; Lorkowski, S.; Li, Z.; Zhou, X.; Cullen, P.; Assmann, G. ATP binding cassette transporter ABCA1 modulates the secretion of apolipoprotein E from human monocyte-derived macrophages. FASEB J 2001, 15, 1555-61.
- Wang, N.; Silver, D. L.; Thiele, C.; Tall, A. R. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 2001, 276, 23742-7.
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A. T.; Neve, B.; Torra, I. P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; Brewer, H. B.; Fruchart, J. C.; Clavey, V.; Staels, B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med 2001, 7, 53-8.
- Fielding, P. E.; Nagao, K.; Hakamata, H.; Chimini, G.; Fielding, C. J. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry 2000, 39, 14113-20.
- Nieland, T. J.; Chroni, A.; Fitzgerald, M. L.; Maliga, Z.; Zannis, V. I.; Kirchhausen, T.; Krieger, M. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J Lipid Res 2004, 45, 1256-65.
- Lewandowski, C. T.; Khan, M. W.; BenAissa, M.; Dubrovskyi, O.; Ackerman-Berrier, M.; LaDu, M. J.; Layden, B. T.; Thatcher, G. R. J. Metabolomic analysis of a selective ABCA1 inducer in obesogenic challenge provides a rationale for therapeutic development. EBioMedicine 2021, 66, 103287.
- Wang, D.; Hiebl, V.; Schachner, D.; Ladurner, A.; Heiss, E. H.; Atanasov, A. G.; Dirsch, V. M. Soraphen A enhances macrophage cholesterol efflux via indirect LXR activation and ABCA1 upregulation. Biochem Pharmacol 2020, 177, 114022.
- Quach, D.; Vitali, C.; La, F. M.; Xiao, A. X.; Millar, J. S.; Tang, C.; Rader, D. J.; Phillips, M. C.; Lyssenko, N. N. Cell lipid metabolism modulators 2-bromopalmitate, D609, monensin, U18666A and probucol shift discoidal HDL formation to the smaller-sized particles: implications for the mechanism of HDL assembly. Biochim Biophys Acta 2016, 1861, 1968-79.
- de la Llera-Moya, M.; Drazul-Schrader, D.; Asztalos, B. F.; Cuchel, M.; Rader, D. J.; Rothblat, G. H. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 2010, 30, 796-801.
- Arakawa, R.; Tsujita, M.; Iwamoto, N.; Ito-Ohsumi, C.; Lu, R.; Wu, C. A.; Shimizu, K.; Aotsuka, T.; Kanazawa, H.; Abe-Dohmae, S.; Yokoyama, S. Pharmacological inhibition of ABCA1 degradation increases HDL biogenesis and exhibits antiatherogenesis. J Lipid Res 2009, 50, 2299-305.
- Adorni, M. P.; Zimetti, F.; Billheimer, J. T.; Wang, N.; Rader, D. J.; Phillips, M. C.; Rothblat, G. H. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 2007, 48, 2453-62.
- Duong, M.; Collins, H. L.; Jin, W.; Zanotti, I.; Favari, E.; Rothblat, G. H. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler Thromb Vasc Biol 2006, 26, 541-7.
- Favari, E.; Zanotti, I.; Zimetti, F.; Ronda, N.; Bernini, F.; Rothblat, G. H. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol 2004, 24, 2345-50.
- Shinohara, M.; Shinohara, M.; Zhao, J.; Fu, Y.; Liu, C. C.; Kanekiyo, T.; Bu, G. 5-HT3 Antagonist Ondansetron Increases apoE Secretion by Modulating the LXR-ABCA1 Pathway. Int J Mol Sci 2019, 20.
- Wang, W.; Nakashima, K. I.; Hirai, T.; Inoue, M. Neuroprotective effect of naturally occurring RXR agonists isolated from Sophora tonkinensis Gagnep. on amyloid-beta-induced cytotoxicity in PC12 cells. J Nat Med 2019, 73, 154-62.
- Kheirollah, A.; Ito, J.; Nagayasu, Y.; Lu, R.; Yokoyama, S. Cyclosporin A inhibits apolipoprotein A-I-induced early events in cellular cholesterol homeostasis in rat astrocytes. Neuropharmacol 2006, 51, 693-700.
- Palmer, M. A.; Smart, E.; Haslam, I. S. Localisation and regulation of cholesterol transporters in the human hair follicle: mapping changes across the hair cycle. Histochem Cell Biol 2021, 155, 529-45.
- Bardin, E.; Pastor, A.; Semeraro, M.; Golec, A.; Hayes, K.; Chevalier, B.; Berhal, F.; Prestat, G.; Hinzpeter, A.; Gravier-Pelletier, C.; Pranke, I.; Sermet-Gaudelus, I. Modulators of CFTR. Updates on clinical development and future directions. Eur J Med Chem 2021, 213, 113195.
- Schmitt, S. M.; Stefan, K.; Wiese, M. Pyrrolopyrimidine derivatives and purine analogs as novel activators of Multidrug Resistance-associated Protein 1 (MRP1, ABCC1). Biochim Biophys Acta 2017, 1859, 69-79.
- Trechot, P.; Conart, J. B.; Trechot, F. ATP sensitive potassium channel openers: A new class of ocular hypotensive agents. Exp Eye Res 2019, 178, 223-4.
- Csandl, M. A.; Conseil, G.; Cole, S. P. cysteinyl leukotriene receptor 1/2 antagonists nonselectively modulate organic anion transport by multidrug resistance proteins (MRP1-4). Drug Metab Dispos 2016, 44, 857-66.
- Wang, H.; Tang, Y.; Wang, L.; Long, C. L.; Zhang, Y. L. ATP-sensitive potassium channel openers and 2,3-dimethyl-2-butylamine derivatives. Curr Med Chem 2007, 14, 133-55.
- Moreau, C.; Prost, A. L.; Derand, R.; Vivaudou, M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol 2005, 38, 951-63.
- Bielicki, J. K.; Zhang, H.; Cortez, Y.; Zheng, Y.; Narayanaswami, V.; Patel, A.; Johansson, J.; Azhar, S. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res 2010, 51, 1496-503.
- Natarajan, P.; Forte, T. M.; Chu, B.; Phillips, M. C.; Oram, J. F.; Bielicki, J. K. Identification of an apolipoprotein A-I structural element that mediates cellular cholesterol efflux and stabilizes ATP binding cassette transporter A1. J Biol Chem 2004, 279, 24044-52.
- Vedhachalam, C.; Narayanaswami, V.; Neto, N.; Forte, T. M.; Phillips, M. C.; Lund-Katz, S.; Bielicki, J. K. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry 2007, 46, 2583-93.
- Hafiane, A.; Bielicki, J. K.; Johansson, J. O.; Genest, J. Novel APOE-derived ABCA1 agonist peptide (CS-6253) promotes reverse cholesterol transport and induces formation of prebeta-1 HDL In Vitro. PloS one 2015, 10, e0131997.
- Boehm-Cagan, A.; Bar, R.; Liraz, O.; Bielicki, J. K.; Johansson, J. O.; Michaelson, D. M. ABCA1 agonist reverses the APOE4-driven cognitive and brain pathologies. J Alzheimers Dis 2016, 54, 1219-33.
- Wang, L.; Schuster, G. U.; Hultenby, K.; Zhang, Q.; Andersson, S.; Gustafsson, J. A. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci U S A 2002, 99, 13878-83.
- Santamarina-Fojo, S.; Remaley, A. T.; Neufeld, E. B.; Brewer, H. B., Jr. Regulation and intracellular trafficking of the ABCA1 transporter. J Lipid Res 2001, 42, 1339-45.
- Repa, J. J.; Turley, S. D.; Lobaccaro, J. A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R. A.; Dietschy, J. M.; Mangelsdorf, D. J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 2000, 289, 1524-9.
- Cramer, P. E.; Cirrito, J. R.; Wesson, D. W.; Lee, C. Y.; Karlo, J. C.; Zinn, A. E.; Casali, B. T.; Restivo, J. L.; Goebel, W. D.; James, M. J.; Brunden, K. R.; Wilson, D. A.; Landreth, G. E. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 2012, 335, 1503-6.
- Terwel, D.; Steffensen, K. R.; Verghese, P. B.; Kummer, M. P.; Gustafsson, J. A.; Holtzman, D. M.; Heneka, M. T. Critical role of astroglial apolipoprotein E and liver X receptor-alpha expression for microglial Abeta phagocytosis. J Neurosci 2011, 31, 7049-59.
- Lefterov, I.; Bookout, A.; Wang, Z.; Staufenbiel, M.; Mangelsdorf, D.; Koldamova, R. Expression profiling in APP23 mouse brain: inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Mol Neurodegener 2007, 2, 20.
- Riddell, D. R.; Zhou, H.; Comery, T. A.; Kouranova, E.; Lo, C. F.; Warwick, H. K.; Ring, R. H.; Kirksey, Y.; Aschmies, S.; Xu, J.; Kubek, K.; Hirst, W. D.; Gonzales, C.; Chen, Y.; Murphy, E.; Leonard, S.; Vasylyev, D.; Oganesian, A.; Martone, R. L.; Pangalos, M. N.; Reinhart, P. H.; Jacobsen, J. S. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol Cell Neurosci 2007, 34, 621-8.
- Koldamova, R. P.; Lefterov, I. M.; Staufenbiel, M.; Wolfe, D.; Huang, S.; Glorioso, J. C.; Walter, M.; Roth, M. G.; Lazo, J. S. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J Biol Chem 2005, 280, 4079-88.
- Donkin, J. J.; Stukas, S.; Hirsch-Reinshagen, V.; Namjoshi, D.; Wilkinson, A.; May, S.; Chan, J.; Fan, J.; Collins, J.; Wellington, C. L. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J Biol Chem 2010, 285, 34144-54.
- Vanmierlo, T.; Rutten, K.; Dederen, J.; Bloks, V. W.; van Vark-van der Zee, L. C.; Kuipers, F.; Kiliaan, A.; Blokland, A.; Sijbrands, E. J.; Steinbusch, H.; Prickaerts, J.; Lutjohann, D.; Mulder, M. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol Aging 2011, 32, 1262-72.
- Bunay, J.; Fouache, A.; Trousson, A.; de Joussineau, C.; Bouchareb, E.; Zhu, Z.; Kocer, A.; Morel, L.; Baron, S.; Lobaccaro, J. A. Screening for liver X receptor modulators: Where are we and for what use? Br J Pharmacol 2020, 178, 3277-93.
- Salonurmi, T.; Nabil, H.; Ronkainen, J.; Hyotylainen, T.; Hautajarvi, H.; Savolainen, M. J.; Tolonen, A.; Oresic, M.; Kansakoski, P.; Rysa, J.; Hakkola, J.; Hukkanen, J. 4beta-Hydroxycholesterol signals from the liver to regulate peripheral cholesterol transporters. Front Pharmacol 2020, 11, 361.
- Vaidya, M.; Jentsch, J. A.; Peters, S.; Keul, P.; Weske, S.; Graler, M. H.; Mladenov, E.; Iliakis, G.; Heusch, G.; Levkau, B. Regulation of ABCA1-mediated cholesterol efflux by sphingosine-1-phosphate signaling in macrophages. J Lipid Res 2019, 60, 506-15.
- Castro Navas, F. F.; Giorgi, G.; Maggioni, D.; Pacciarini, M.; Russo, V.; Marinozzi, M. C24-hydroxylated stigmastane derivatives as Liver X Receptor agonists. Chem Phys Lipids 2018, 212, 44-50.
- Hoang, M. H.; Jia, Y.; Jun, H. J.; Lee, J. H.; Lee, B. Y.; Lee, S. J. Fucosterol is a selective liver X receptor modulator that regulates the expression of key genes in cholesterol homeostasis in macrophages, hepatocytes, and intestinal cells. J Agric Food Chem 2012, 60, 11567-75.
- Gao, J.; Xu, Y.; Yang, Y.; Yang, Y.; Zheng, Z.; Jiang, W.; Hong, B.; Yan, X.; Si, S. Identification of upregulators of human ATP-binding cassette transporter A1 via high-throughput screening of a synthetic and natural compound library. J Biomol Screen 2008, 13, 648-56.
- Huang, T. H.; Razmovski-Naumovski, V.; Salam, N. K.; Duke, R. K.; Tran, V. H.; Duke, C. C.; Roufogalis, B. D. A novel LXR-alpha activator identified from the natural product Gynostemma pentaphyllum. Biochem Pharmacol 2005, 70, 1298-308.
- Agassandian, M.; Mathur, S. N.; Zhou, J.; Field, F. J.; Mallampalli, R. K. Oxysterols trigger ABCA1-mediated basolateral surfactant efflux. Am J Respir Cell Mol Biol 2004, 31, 227-33.
- Sone, H.; Shimano, H.; Shu, M.; Nakakuki, M.; Takahashi, A.; Sakai, M.; Sakamoto, Y.; Yokoo, T.; Matsuzaka, K.; Okazaki, H.; Nakagawa, Y.; Iida, K. T.; Suzuki, H.; Toyoshima, H.; Horiuchi, S.; Yamada, N. Statins downregulate ATP-binding-cassette transporter A1 gene expression in macrophages. Biochem Biophys Res Commun 2004, 316, 790-4.
- Zhang, Y.; Beyer, T. P.; Bramlett, K. S.; Yao, S.; Burris, T. P.; Schmidt, R. J.; Eacho, P. I.; Cao, G. Liver X receptor and retinoic X receptor mediated ABCA1 regulation and cholesterol efflux in macrophage cells-messenger RNA measured by branched DNA technology. Mol Genet Metab 2002, 77, 150-8.
- Gan, X.; Kaplan, R.; Menke, J. G.; MacNaul, K.; Chen, Y.; Sparrow, C. P.; Zhou, G.; Wright, S. D.; Cai, T. Q. Dual mechanisms of ABCA1 regulation by geranylgeranyl pyrophosphate. J Biol Chem 2001, 276, 48702-8.
- Chawla, A.; Boisvert, W. A.; Lee, C. H.; Laffitte, B. A.; Barak, Y.; Joseph, S. B.; Liao, D.; Nagy, L.; Edwards, P. A.; Curtiss, L. K.; Evans, R. M.; Tontonoz, P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 2001, 7, 161-71.
- Arakawa, R.; Yokoyama, S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem 2002, 277, 22426-9.
- Di, D.; Wang, Z.; Liu, Y.; Luo, G.; Shi, Y.; Berggren-Soderlund, M.; Nilsson-Ehle, P.; Zhang, X.; Xu, N. ABCA1 upregulating apolipoproein M expression mediates via the RXR/LXR pathway in HepG2 cells. Biochem Biophys Res Commun 2012, 421, 152-6.
- Zanotti, I.; Favari, E.; Sposito, A. C.; Rothblat, G. H.; Bernini, F. Pitavastatin increases ABCA1-mediated lipid efflux from Fu5AH rat hepatoma cells. Biochem Biophys Res Commun 2004, 321, 670-4.
- Ben Aissa, M.; Lewandowski, C. T.; Ratia, K. M.; Lee, S. H.; Layden, B. T.; LaDu, M. J.; Thatcher, G. R. J. Discovery of nonlipogenic ABCA1 inducing compounds with potential in alzheimer's disease and type 2 diabetes. ACS Pharmacol Transl Sci 2021, 4, 143-54.
- Ma, L.; Wang, L.; Nelson, A. T.; Han, C.; He, S.; Henn, M. A.; Menon, K.; Chen, J. J.; Baek, A. E.; Vardanyan, A.; Shahoei, S. H.; Park, S.; Shapiro, D. J.; Nanjappa, S. G.; Nelson, E. R. 27-Hydroxycholesterol acts on myeloid immune cells to induce T cell dysfunction, promoting breast cancer progression. Cancer Lett 2020, 493, 266-83.
- Ray, A. G.; Choudhury, K. R.; Chakraborty, S.; Chakravarty, D.; Chander, V.; Jana, B.; Siddiqui, K. N.; Bandyopadhyay, A. Novel mechanism of cholesterol transport by ABCA5 in macrophages and its role in dyslipidemia. J Mol Biol 2020, 432, 4922-41.
- He, P.; Smith, A.; Gelissen, I. C.; Ammit, A. J. The effect of statins and the synthetic LXR agonist T0901317 on expression of ABCA1 transporter protein in human lung epithelial cell lines in vitro. Pharmacol Rep: PR 2019, 71, 1219-26.
- Leger-Charnay, E.; Masson, E. A. Y.; Morala, T.; Martine, L.; Buteau, B.; Leclere, L.; Bretillon, L.; Gambert, S. Is 24(S)-hydroxycholesterol a potent modulator of cholesterol metabolism in Muller cells? An in vitro study about neuron to glia communication in the retina. Exp Eye Res 2019, 189, 107857.
- Wu, C. A.; Wang, N.; Zhao, D. H. An evaluation of the mechanism of ABCA7 on cellular lipid release in ABCA7-HEC293 cell. Chin Med J 2013, 126, 306-10.
- Lee, J. S.; Kim, E.; Han, S.; Kang, K. L.; Heo, J. S. Evaluating the oxysterol combination of 22(S)-hydroxycholesterol and 20(S)-hydroxycholesterol in periodontal regeneration using periodontal ligament stem cells and alveolar bone healing models. Stem Cell Res Ther 2017, 8, 276.
- Panzenboeck, U.; Kratzer, I.; Sovic, A.; Wintersperger, A.; Bernhart, E.; Hammer, A.; Malle, E.; Sattler, W. Regulatory effects of synthetic liver X receptor- and peroxisome-proliferator activated receptor agonists on sterol transport pathways in polarized cerebrovascular endothelial cells. Int J Biochem Cell Biol 2006, 38, 1314-29.
- Ruan, X. Z.; Moorhead, J. F.; Fernando, R.; Wheeler, D. C.; Powis, S. H.; Varghese, Z. PPAR agonists protect mesangial cells from interleukin 1beta-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J Am Soc Nephrol 2003, 14, 593-600.
- Kim, B. Y.; Son, Y.; Cho, H. R.; Lee, D.; Eo, S. K.; Kim, K. 27-Hydroxycholesterol induces macrophage gene expression via LXR-dependent and -independent mechanisms. Korean J Physiol Pharmacol 2021, 25, 111-8.
- Costet, P.; Lalanne, F.; Gerbod-Giannone, M. C.; Molina, J. R.; Fu, X.; Lund, E. G.; Gudas, L. J.; Tall, A. R. Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol Cell Biol 2003, 23, 7756-66.
- Zhang, C. J.; Zhu, N.; Long, J.; Wu, H. T.; Wang, Y. X.; Liu, B. Y.; Liao, D. F.; Qin, L. Celastrol induces lipophagy via the LXRalpha/ABCA1 pathway in clear cell renal cell carcinoma. Acta Pharmacol Sin 2020, 42, 1472-85.
- Munir, M. T.; Ponce, C.; Santos, J. M.; Sufian, H. B.; Al-Harrasi, A.; Gollahon, L. S.; Hussain, F.; Rahman, S. M. VD3 and LXR agonist (T0901317) combination demonstrated greater potency in inhibiting cholesterol accumulation and inducing apoptosis via ABCA1-CHOP-BCL-2 cascade in MCF-7 breast cancer cells. Mol Biol Rep 2020, 47, 7771-82.
- Shi, Y.; Jiang, S.; Zhao, T.; Gong, Y.; Liao, D.; Qin, L. Celastrol suppresses lipid accumulation through LXRalpha/ABCA1 signaling pathway and autophagy in vascular smooth muscle cells. Biochem Biophys Res Commun 2020, 532, 466-74.
- Warde, K. M.; Schoenmakers, E.; Ribes Martinez, E.; Lim, Y. J.; Leonard, M.; Lawless, S. J.; O'Shea, P.; Chatterjee, K. V.; Gurnell, M.; Hantel, C.; Dennedy, M. C. Liver X receptor inhibition potentiates mitotane-induced adrenotoxicity in ACC. Endocr Relat Cancer 2020, 27, 361-73.
- Wu, G.; Wang, Q.; Xu, Y.; Li, J.; Zhang, H.; Qi, G.; Xia, Q. Targeting the transcription factor receptor LXR to treat clear cell renal cell carcinoma: agonist or inverse agonist? Cell Death Dis 2019, 10, 416.
- Jin, P.; Bian, Y.; Wang, K.; Cong, G.; Yan, R.; Sha, Y.; Ma, X.; Zhou, J.; Yuan, Z.; Jia, S. Homocysteine accelerates atherosclerosis via inhibiting LXRalpha-mediated ABCA1/ABCG1-dependent cholesterol efflux from macrophages. Life Sci 2018, 214, 41-50.
- Luo, G.; Qian, Z.; Qiu, R.; You, Q.; Xiang, H. Lipid reducing activity of novel cholic acid (CA) analogs: Design, synthesis and preliminary mechanism study. Bioorg Chem 2018, 80, 396-407.
- Ni, J.; Zhou, L. L.; Ding, L.; Zhang, X. Q.; Zhao, X.; Li, H.; Cao, H.; Liu, S.; Wang, Z.; Ma, R.; Wu, J.; Feng, J. Efatutazone and T0901317 exert synergistically therapeutic effects in acquired gefitinib-resistant lung adenocarcinoma cells. Cancer Med 2018, 7, 1955-66.
- Saenz, J.; Alba, G.; Reyes-Quiroz, M. E.; Geniz, I.; Jimenez, J.; Sobrino, F.; Santa-Maria, C. Curcumin enhances LXRalpha in an AMP-activated protein kinase-dependent manner in human macrophages. J Nutr Biochem 2018, 54, 48-56.
- Saenz, J.; Santa-Maria, C.; Reyes-Quiroz, M. E.; Geniz, I.; Jimenez, J.; Sobrino, F.; Alba, G. grapefruit flavonoid naringenin regulates the expression of lxralpha in THP-1 macrophages by modulating AMP-activated protein kinase. Mol Pharm 2018, 15, 1735-45.
- Lei, C.; Lin, R.; Wang, J.; Tao, L.; Fu, X.; Qiu, Y.; Lei, B. Amelioration of amyloid beta-induced retinal inflammatory responses by a LXR agonist TO901317 is associated with inhibition of the NF-kappaB signaling and NLRP3 inflammasome. Neurosci 2017, 360, 48-60.
- Fernandez-Suarez, M. E.; Escola-Gil, J. C.; Pastor, O.; Davalos, A.; Blanco-Vaca, F.; Lasuncion, M. A.; Martinez-Botas, J.; Gomez-Coronado, D. Clinically used selective estrogen receptor modulators affect different steps of macrophage-specific reverse cholesterol transport. Sci Rep 2016, 6, 32105.
- Hoang, M. H.; Jia, Y.; Jun, H. J.; Lee, J. H.; Lee, D. H.; Hwang, B. Y.; Kim, W. J.; Lee, H. J.; Lee, S. J. Ethyl 2,4,6-trihydroxybenzoate is an agonistic ligand for liver X receptor that induces cholesterol efflux from macrophages without affecting lipid accumulation in HepG2 cells. Bioorg Med Chem Lett 2012, 22, 4094-9.
- Maejima, T.; Sugano, T.; Yamazaki, H.; Yoshinaka, Y.; Doi, T.; Tanabe, S.; Nishimaki-Mogami, T. Pitavastatin increases ABCA1 expression by dual mechanisms: SREBP2-driven transcriptional activation and PPARalpha-dependent protein stabilization but without activating LXR in rat hepatoma McARH7777 cells. J Pharmacol Sci 2011, 116, 107-15.
- Fitz, N. F.; Cronican, A.; Pham, T.; Fogg, A.; Fauq, A. H.; Chapman, R.; Lefterov, I.; Koldamova, R. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J Neurosci 2010, 30, 6862-72.
- Fukumoto, H.; Deng, A.; Irizarry, M. C.; Fitzgerald, M. L.; Rebeck, G. W. Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Abeta levels. J Biol Chem 2002, 277, 48508-13.
- van Riel, N. A. W.; Tiemann, C. A.; Hilbers, P. A. J.; Groen, A. K. Metabolic modeling combined with machine learning integrates longitudinal data and identifies the origin of LXR-induced hepatic steatosis. Front Bioeng Biotechnol 2020, 8, 536957.
- Chisholm, J. W.; Hong, J.; Mills, S. A.; Lawn, R. M. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J Lipid Res 2003, 44, 2039-48.
- Liang, Z.; Gu, T.; Wang, J.; She, J.; Ye, Y.; Cao, W.; Luo, X.; Xiao, J.; Liu, Y.; Tang, L.; Zhou, X. Chromene and chromone derivatives as liver X receptors modulators from a marine-derived Pestalotiopsis neglecta fungus. Bioorg Chem 2021, 112, 104927.
- Wang, J.; Liang, Z.; Li, K.; Yang, B.; Liu, Y.; Fang, W.; Tang, L.; Zhou, X. Ene-yne hydroquinones from a marine-derived strain of the fungus pestalotiopsis neglecta with effects on liver X receptor alpha. J Nat Prod 2020, 83, 1258-64.
- Chen, Y.; Wang, Y.; Yang, M.; Guo, M. Y. Allicin inhibited staphylococcus aureus -induced mastitis by reducing lipid raft stability via lxralpha in mice. J Agric Food Chem 2019, 67, 10863-70.
- Grinman, D. Y.; Careaga, V. P.; Wellberg, E. A.; Dansey, M. V.; Kordon, E. C.; Anderson, S. M.; Maier, M. S.; Burton, G.; MacLean, P. S.; Rudolph, M. C.; Pecci, A. Liver X receptor-alpha activation enhances cholesterol secretion in lactating mammary epithelium. American journal of physiology. Endocrinol Metab 2019, 316, E1136-E1145.
- Huang, Y.; Liu, H.; Zhang, Y.; Li, J.; Wang, C.; Zhou, L.; Jia, Y.; Li, X. Synthesis and biological evaluation of ginsenoside compound K derivatives as a novel class of LXRalpha activator. Molecules 2017, 22, 1232.
- Liu, B.; He, Z.; Wang, J.; Xin, Z.; Wang, J.; Li, F.; Fu, Y. Taraxasterol inhibits lPS-induced inflammatory response in BV2 microglia cells by activating LXRalpha. Front Pharmacol 2018, 9, 278.
- Biswas, L.; Zeng, Z.; Graham, A.; Shu, X. Gypenosides mediate cholesterol efflux and suppress oxidized LDL induced inflammation in retinal pigment epithelium cells. Exp Eye Res 2020, 191, 107931.
- Fu, Y.; Xin, Z.; Liu, B.; Wang, J.; Wang, J.; Zhang, X.; Wang, Y.; Li, F. Platycodin D inhibits inflammatory response in lPS-stimulated primary rat microglia cells through activating LXRalpha-ABCA1 signaling pathway. Front Immunol 2017, 8, 1929.
- Shuai-Cheng, W.; Xiu-Ling, C.; Jian-Qing, S.; Zong-Mei, W.; Zhen-Jiang, Y.; Lian-Tao, L. Saikosaponin A protects chickens against pullorum disease via modulation of cholesterol. Poult Sci 2019, 98, 3539-47.
- Kilby, E. L.; Kelly, D. M.; Jones, T. H. Testosterone stimulates cholesterol clearance from human macrophages by activating LXRalpha. Life Sci 2021, 269, 119040.
- Li, S.; Cao, H.; Shen, D.; Jia, Q.; Chen, C.; Xing, S. L. Quercetin protects against oxLDLinduced injury via regulation of ABCAl, LXRalpha and PCSK9 in RAW264.7 macrophages. Mol Med Rep 2018, 18, 799-806.
- Malar, D. S.; Suryanarayanan, V.; Prasanth, M. I.; Singh, S. K.; Balamurugan, K.; Devi, K. P. Vitexin inhibits Abeta25-35 induced toxicity in Neuro-2a cells by augmenting Nrf-2/HO-1 dependent antioxidant pathway and regulating lipid homeostasis by the activation of LXR-alpha. Toxicol In Vitro 2018, 50, 160-171.
- Xu, X.; Lei, T.; Li, W.; Ou, H. Enhanced cellular cholesterol efflux by naringenin is mediated through inhibiting endoplasmic reticulum stress - ATF6 activity in macrophages. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 1472-82.
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARgamma, LXRalpha and ABCA1. Int J Mol Med 2019, 44, 893-902.
- Li, S. S.; Cao, H.; Shen, D. Z.; Chen, C.; Xing, S. L.; Dou, F. F.; Jia, Q. L. Effect of quercetin on atherosclerosis based on expressions of ABCA1, LXR-alpha and PCSK in APOE(-/-) mice. Chin J Integr Med 2020, 26, 114-21.
- Teng, I. J.; Tsai, M. C.; Shih, S. F.; Tsuei, B. F.; Chang, H.; Chuang, Y. P.; Lin, C. S.; Chern, C. Y.; Chen, S. J. Chalcone derivatives enhance ATP-binding cassette transporters A1 in human THP-1 macrophages. Molecules 2018, 23, 1620.
- Chen, L. W.; Tsai, M. C.; Chern, C. Y.; Tsao, T. P.; Lin, F. Y.; Chen, S. J.; Tsui, P. F.; Liu, Y. W.; Lu, H. J.; Wu, W. L.; Lin, W. S.; Tsai, C. S.; Lin, C. S. A chalcone derivative, 1m-6, exhibits atheroprotective effects by increasing cholesterol efflux and reducing inflammation-induced endothelial dysfunction. Br J Pharmacol 2020, 177, 5375-92.
- Liu, X. X.; Zhang, X. W.; Wang, K.; Wang, X. Y.; Ma, W. L.; Cao, W.; Mo, D.; Sun, Y.; Li, X. Q. Kuwanon G attenuates atherosclerosis by upregulation of LXRalpha-ABCA1/ABCG1 and inhibition of NFkappaB activity in macrophages. Toxicol Appl Pharmacol 2018, 341, 56-63.
- Wang, G.; Gao, J. H.; He, L. H.; Yu, X. H.; Zhao, Z. W.; Zou, J.; Wen, F. J.; Zhou, L.; Wan, X. J.; Tang, C. K. Fargesin alleviates atherosclerosis by promoting reverse cholesterol transport and reducing inflammatory response. Biochim Biophys Acta Mol Cell Biol Lipids 2020, 1865, 158633.
- Xu, Y.; Liu, Q.; Xu, Y.; Liu, C.; Wang, X.; He, X.; Zhu, N.; Liu, J.; Wu, Y.; Li, Y.; Li, N.; Feng, T.; Lai, F.; Zhang, M.; Hong, B.; Jiang, J. D.; Si, S. Rutaecarpine suppresses atherosclerosis in ApoE-/- mice through upregulating ABCA1 and SR-BI within RCT. J Lipid Res 2014, 55, 1634-47.
- Pattanayak, S. P.; Bose, P.; Sunita, P.; Siddique, M. U. M.; Lapenna, A. Bergapten inhibits liver carcinogenesis by modulating LXR/PI3K/Akt and IDOL/LDLR pathways. Biomed Pharmacother 2018, 108, 297-308.
- Shi, X.; Zhang, Y.; Lin, B.; Zhou, Y.; Suo, W.; Wei, J.; Zhang, L.; Lin, J.; Xiao, F.; Zhao, L.; Lin, Y. Danthron attenuates experimental atherosclerosis by targeting foam cell formation. Exp Physiol 2021, 106, 653-62.
- Wang, W.; Zhang, Z. Z.; Wu, Y.; Wang, R. Q.; Chen, J. W.; Chen, J.; Zhang, Y.; Chen, Y. J.; Geng, M.; Xu, Z. D.; Dai, M.; Li, J. H.; Pan, L. L. (-)-epigallocatechin-3-gallate ameliorates atherosclerosis and modulates hepatic lipid metabolic gene expression in apolipoprotein E knockout mice: Involvement of TTC39B. Front Pharmacol 2018, 9, 195.
- Zheng, S.; Li, L.; Li, N.; Du, Y.; Zhang, N. 1, 6-O, O-diacetylbritannilactone from inula britannica induces anti-tumor effect on oral squamous cell carcinoma via miR-1247-3p/LXRalpha/ABCA1 Signaling. Onco Targets Ther 2020, 13, 11097-109.
- Mustra Rakic, J.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, C. O.; Ausman, L. M.; Wang, X. D. Lycopene inhibits smoke-induced chronic obstructive pulmonary disease and lung carcinogenesis by modulating reverse cholesterol transport in ferrets. Cancer Prev Res (Phila) 2019, 12, 421-32.
- Gwon, M. H.; Im, Y. S.; Seo, A. R.; Kim, K. Y.; Moon, H. R.; Yun, J. M. Phenethyl isothiocyanate protects against high fat/cholesterol diet-induced obesity and atherosclerosis in C57BL/6 mice. Nutrients 2020, 12, 3657.
- Chen, Y.; Zhao, Y. F.; Yang, J.; Jing, H. Y.; Liang, W.; Chen, M. Y.; Yang, M.; Wang, Y.; Guo, M. Y. Selenium alleviates lipopolysaccharide-induced endometritis via regulating the recruitment of TLR4 into lipid rafts in mice. Food Funct 2020, 11, 200-10.
- Shen, D.; Zhao, D.; Yang, X.; Zhang, J.; He, H.; Yu, C. Geniposide against atherosclerosis by inhibiting the formation of foam cell and lowering reverse lipid transport via p38/MAPK signaling pathways. Eur J Pharmacol 2019, 864, 172728.
- Tsunemi, A.; Ueno, T.; Fukuda, N.; Watanabe, T.; Tahira, K.; Haketa, A.; Hatanaka, Y.; Tanaka, S.; Matsumoto, T.; Matsumoto, Y.; Nagase, H.; Soma, M. A novel gene regulator, pyrrole-imidazole polyamide targeting ABCA1 gene increases cholesterol efflux from macrophages and plasma HDL concentration. J Mol Med (Berl) 2014, 92, 509-21.
- Ma, X.; Li, S. F.; Qin, Z. S.; Ye, J.; Zhao, Z. L.; Fang, H. H.; Yao, Z. W.; Gu, M. N.; Hu, Y. W. Propofol up-regulates expression of ABCA1, ABCG1, and SR-B1 through the PPARgamma/LXRalpha signaling pathway in THP-1 macrophage-derived foam cells. Cardiovasc Pathol 2015, 24, 230-5.
- Belorusova, A. Y.; Evertsson, E.; Hovdal, D.; Sandmark, J.; Bratt, E.; Maxvall, I.; Schulman, I. G.; Akerblad, P.; Lindstedt, E. L. Structural analysis identifies an escape route from the adverse lipogenic effects of liver X receptor ligands. Commun Biol 2019, 2, 431.
- Liu, H.; Jiang, X.; Gao, X.; Tian, W.; Xu, C.; Wang, R.; Xu, Y.; Wei, L.; Cao, F.; Li, W. Identification of N-benzothiazolyl-2-benzenesulfonamides as novel ABCA1 expression upregulators. RSC Med Chem 2020, 11, 411-8.
- Ren, G.; Bao, W.; Zeng, Z.; Zhang, W.; Shang, C.; Wang, M.; Su, Y.; Zhang, X. K.; Zhou, H. Retinoid X receptor alpha nitro-ligand Z-10 and its optimized derivative Z-36 reduce beta-amyloid plaques in Alzheimer's disease mouse model. Mol Pharm 2019, 16, 480-8.
- Li, Y.; Feng, T.; Liu, P.; Liu, C.; Wang, X.; Li, D.; Li, N.; Chen, M.; Xu, Y.; Si, S. Optimization of rutaecarpine as ABCA1 up-regulator for treating atherosclerosis. ACS Med Chem Lett 2014, 5, 884-8.
- Singh, S. B.; Ondeyka, J. G.; Liu, W.; Chen, S.; Chen, T. S.; Li, X.; Bouffard, A.; Dropinski, J.; Jones, A. B.; McCormick, S.; Hayes, N.; Wang, J.; Sharma, N.; Macnaul, K.; Hernandez, M.; Chao, Y. S.; Baffic, J.; Lam, M. H.; Burton, C.; Sparrow, C. P.; Menke, J. G. Discovery and development of dimeric podocarpic acid leads as potent agonists of liver X receptor with HDL cholesterol raising activity in mice and hamsters. Bioorg Med Chem Lett 2005, 15, 2824-8.
- Boehm-Cagan, A.; Michaelson, D. M. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neurosci 2014, 34, 7293-301.
- Niesor, E. J.; Schwartz, G. G.; Perez, A.; Stauffer, A.; Durrwell, A.; Bucklar-Suchankova, G.; Benghozi, R.; Abt, M.; Kallend, D. Statin-induced decrease in ATP-binding cassette transporter A1 expression via microRNA33 induction may counteract cholesterol efflux to high-density lipoprotein. Cardiovasc Drugs Ther 2015, 29, 7-14.
- Allen, A. M.; Graham, A. Mitochondrial function is involved in regulation of cholesterol efflux to apolipoprotein (apo)A-I from murine RAW 264.7 macrophages. Lipids Health Dis 2012, 11, 169.
- Abe-Dohmae, S.; Ikeda, Y.; Matsuo, M.; Hayashi, M.; Okuhira, K.; Ueda, K.; Yokoyama, S. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J Biol Chem 2004, 279, 604-11.
- Abe-Dohmae, S.; Suzuki, S.; Wada, Y.; Aburatani, H.; Vance, D. E.; Yokoyama, S. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry 2000, 39, 11092-9.
- Zhong, Y.; Liu, C.; Feng, J.; Li, J. F.; Fan, Z. C. Curcumin affects ox-LDL-induced IL-6, TNF-alpha, MCP-1 secretion and cholesterol efflux in THP-1 cells by suppressing the TLR4/NF-kappaB/miR33a signaling pathway. Exp Ther Med 2020, 20, 1856-70.
- Wang, Y.; Wen, Y.; Xiao, P.; Sun, J.; Chen, M.; Gu, C.; Kong, Y.; Gu, A.; Zhang, J.; Wang, Y. Di-n-butyl phthalate promotes lipid accumulation via the miR200c-5p-ABCA1 pathway in THP-1 macrophages. Environ Pollut 2020, 264, 114723.
- Phang, S. W.; Ooi, B. K.; Ahemad, N.; Yap, W. H. Maslinic acid suppresses macrophage foam cells formation: Regulation of monocyte recruitment and macrophage lipids homeostasis. Vascul Pharmacol 2020, 128-129, 106675.
- Lou, K.; Huang, P.; Ma, H.; Wang, X.; Xu, H.; Wang, W. Orlistat increases arsenite tolerance in THP-1 derived macrophages through the up-regulation of ABCA1. Drug Chem Toxicol 2019, 1-9.
- Chen, C. H.; Zhao, J. F.; Hsu, C. P.; Kou, Y. R.; Lu, T. M.; Lee, T. S. The detrimental effect of asymmetric dimethylarginine on cholesterol efflux of macrophage foam cells: Role of the NOX/ROS signaling. Free Radic Biol Med 2019, 143, 354-65.
- Zhao, W.; Wang, L.; Haller, V.; Ritsch, A. A novel candidate for prevention and treatment of atherosclerosis: Urolithin B decreases lipid plaque deposition in APOE(-/-) mice and increases early stages of reverse cholesterol transport in OX-LDL treated macrophages cells. Mol Nutr Food Res 2019, 63, e1800887.
- Zhong, Y.; Feng, J.; Fan, Z.; Li, J. Curcumin increases cholesterol efflux via heme oxygenase1mediated ABCA1 and SRBI expression in macrophages. Mol Med Rep 2018, 17, 6138-43.
- Wang, L.; Wesemann, S.; Krenn, L.; Ladurner, A.; Heiss, E. H.; Dirsch, V. M.; Atanasov, A. G. Erythrodiol, an olive oil constituent, increases the half-life of ABCA1 and enhances cholesterol efflux from THP-1-derived macrophages. Front Pharmacol 2017, 8, 375.
- Kemmerer, M.; Wittig, I.; Richter, F.; Brune, B.; Namgaladze, D. AMPK activates LXRalpha and ABCA1 expression in human macrophages. Int J Biochem Cell Biol 2016, 78, 1-9.
- Li, X. Y.; Kong, L. X.; Li, J.; He, H. X.; Zhou, Y. D. Kaempferol suppresses lipid accumulation in macrophages through the downregulation of cluster of differentiation 36 and the upregulation of scavenger receptor class B type I and ATP-binding cassette transporters A1 and G1. Int J Mol Med 2013, 31, 331-8.
- Seneviratne, A.; Cave, L.; Hyde, G.; Moestrup, S. K.; Carling, D.; Mason, J. C.; Haskard, D. O.; Boyle, J. J. Metformin directly suppresses atherosclerosis in normoglycaemic mice via haematopoietic adenosine monophosphate-activated protein kinase. Cardiovasc Res 2021, 117, 1295-308.
- Lu, S.; Luo, Y.; Sun, G.; Sun, X. ginsenoside compound K attenuates OX-LDL-mediated macrophage inflammation and foam cell formation via autophagy induction and modulating NF-kappaB, P38, and JNK MAPK signaling. Front Pharmacol 2020, 11, 567238.
- Wang, M. S.; Han, Q. S.; Jia, Z. R.; Chen, C. S.; Qiao, C.; Liu, Q. Q.; Zhang, Y. M.; Wang, K. W.; Wang, J.; Xiao, K.; Ding, X. S. PPARalpha agonist fenofibrate relieves acquired resistance to gefitinib in non-small cell lung cancer by promoting apoptosis via PPARalpha/AMPK/AKT/FoxO1 pathway. Acta Pharmacol Sin 2021.
- Lundasen, T.; Pedrelli, M.; Bjorndal, B.; Rozell, B.; Kuiper, R. V.; Burri, L.; Pavanello, C.; Turri, M.; Skorve, J.; Berge, R. K.; Alexson, S. E. H.; Tillander, V. The PPAR pan-agonist tetradecylthioacetic acid promotes redistribution of plasma cholesterol towards large HDL. PloS one 2020, 15, e0229322.
- Shen, C. Y.; Lin, J. J.; Jiang, J. G.; Wang, T. X.; Zhu, W. Potential roles of dietary flavonoids from Citrus aurantium L. var. amara Engl. in atherosclerosis development. Food Funct 2020, 11, 561-71.
- Lin, Y.; Ren, N.; Li, S.; Chen, M.; Pu, P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed Pharmacother 2019, 117, 109042.
- Guo, T.; Liu, Q.; Hou, P.; Li, F.; Guo, S.; Song, W.; Zhang, H.; Liu, X.; Zhang, S.; Zhang, J.; Ho, C. T.; Bai, N. Stilbenoids and cannabinoids from the leaves of Cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct 2018, 9, 6608-17.
- Dong, T.; Lyu, J.; Imachi, H.; Kobayashi, T.; Fukunaga, K.; Sato, S.; Ibata, T.; Yoshimoto, T.; Yonezaki, K.; Iwama, H.; Zhang, G.; Murao, K. Selective peroxisome proliferator-activated receptor-alpha modulator K-877 regulates the expression of ATP-binding cassette transporter A1 in pancreatic beta cells. Eur J Pharmacol 2018, 838, 78-84.
- Wang, X.; Luo, J.; Li, N.; Liu, L.; Han, X.; Liu, C.; Zuo, X.; Jiang, X.; Li, Y.; Xu, Y.; Si, S. E3317 promotes cholesterol efflux in macrophage cells via enhancing ABCA1 expression. Biochem Biophys Res Commun 2018, 504, 68-74.
- Silva, J. C.; de Oliveira, E. M.; Turato, W. M.; Trossini, G. H. G.; Maltarollo, V. G.; Pitta, M. G. R.; Pitta, I. R.; de Las Heras, B.; Bosca, L.; Rudnicki, M.; Abdalla, D. S. P. GQ-11: A new PPAR agonist improves obesity-induced metabolic alterations in LDLr(-/-) mice. Int J Obes (Lond) 2018, 42, 1062-72.
- Wang, S.; Zhang, X.; Li, X.; Liu, Q.; Zhou, Y.; Guo, P.; Dong, Z.; Wu, C. Phenylpropanoid glucosides from Tadehagi triquetrum inhibit oxLDL-evoked foam cell formation through modulating cholesterol homeostasis in RAW264.7 macrophages. Nat Prod Res 2019, 33, 893-6.
- Brunham, L. R.; Kruit, J. K.; Pape, T. D.; Timmins, J. M.; Reuwer, A. Q.; Vasanji, Z.; Marsh, B. J.; Rodrigues, B.; Johnson, J. D.; Parks, J. S.; Verchere, C. B.; Hayden, M. R. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007, 13, 340-7.
- Gao, Q.; Wei, A.; Chen, F.; Chen, X.; Ding, W.; Ding, Z.; Wu, Z.; Du, R.; Cao, W. Enhancing PPARgamma by HDAC inhibition reduces foam cell formation and atherosclerosis in ApoE deficient mice. Pharmacol Res 2020, 160, 105059.
- Farzanegan Gharabolagh, A.; Bamdad, T.; Hedayati, M.; Dehghan Manshadi, S. A. the synergistic effect of fluvastatin and IFN-lambda on peripheral blood mononuclear cells of chronic hepatitis C virus (HCV) patients with IL-28B rs12979860 CC Genotype. Iran J Allergy Asthma Immunol 2019, 18, 533-42.
- Wong, J.; Quinn, C. M.; Brown, A. J. Statins inhibit synthesis of an oxysterol ligand for the liver x receptor in human macrophages with consequences for cholesterol flux. Arterioscler Thromb Vasc Biol 2004, 24, 2365-71.
- Pirmoradi, L.; Seyfizadeh, N.; Ghavami, S.; Zeki, A. A.; Shojaei, S. Targeting cholesterol metabolism in glioblastoma: a new therapeutic approach in cancer therapy. J Investig Med 2019, 67, 715-9.
- Fu, Y.; Zhou, E.; Wei, Z.; Song, X.; Liu, Z.; Wang, T.; Wang, W.; Zhang, N.; Liu, G.; Yang, Z. Glycyrrhizin inhibits lipopolysaccharide-induced inflammatory response by reducing TLR4 recruitment into lipid rafts in RAW264.7 cells. Biochim Biophys Acta 2014, 1840, 1755-64.
- Singh, A. B.; Dong, B.; Kraemer, F. B.; Liu, J. FXR activation promotes intestinal cholesterol excretion and attenuates hyperlipidemia in SR-B1-deficient mice fed a high-fat and high-cholesterol diet. Physiol Rep 2020, 8, e14387.
- Yang, Y.; Li, X.; Peng, L.; An, L.; Sun, N.; Hu, X.; Zhou, P.; Xu, Y.; Li, P.; Chen, J. Tanshindiol C inhibits oxidized low-density lipoprotein induced macrophage foam cell formation via a peroxiredoxin 1 dependent pathway. Biochim Biophys Acta Mol Basis Dis 2018, 1864, 882-90.
- Koga, M.; Kanaoka, Y.; Inada, K.; Omine, S.; Kataoka, Y.; Yamauchi, A. Hesperidin blocks varenicline-aggravated atherosclerotic plaque formation in apolipoprotein E knockout mice by downregulating net uptake of oxidized low-density lipoprotein in macrophages. J Pharmacol Sci 2020, 143, 106-11.
- Han, Q. A.; Su, D.; Shi, C.; Liu, P.; Wang, Y.; Zhu, B.; Xia, X. Urolithin A attenuated ox-LDL-induced cholesterol accumulation in macrophages partly through regulating miR-33a and ERK/AMPK/SREBP1 signaling pathways. Food Funct 2020, 11, 3432-40.
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br J Pharmacol 2020, 177, 1754-72.
- Wang, L.; Zhu, J.; Cui, L.; Wang, Q.; Huang, W.; Ji, X.; Yang, Q.; Rui, C. Overexpression of ATP-binding cassette transporters associated with sulfoxaflor resistance in Aphis gossypii glover. Pest Manag Sci 2021, 77, 4064-72.
- Song, Q.; Hu, Z.; Xie, X.; Cai, H. Zafirlukast prevented ox-LDL-induced formation of foam cells. Toxicol Appl Pharmacol 2020, 409, 115295.
- Tomioka, M.; Toda, Y.; Manucat, N. B.; Akatsu, H.; Fukumoto, M.; Kono, N.; Arai, H.; Kioka, N.; Ueda, K. Lysophosphatidylcholine export by human ABCA7. Biochim Biophys Acta Mol Cell Biol Lipids 2017, 1862, 658-65.
- Fan, J.; Zhao, R. Q.; Parro, C.; Zhao, W.; Chou, H. Y.; Robert, J.; Deeb, T. Z.; Raynoschek, C.; Barichievy, S.; Engkvist, O.; Maresca, M.; Hicks, R.; Meuller, J.; Moss, S. J.; Brandon, N. J.; Wood, M. W.; Kulic, I.; Wellington, C. L. Small molecule inducers of ABCA1 and apoE that act through indirect activation of the LXR pathway. J Lipid Res 2018, 59, 830-42.
- Liu, J.; Yang, B.; Wang, Y.; Wu, Y.; Fan, B.; Zhu, S.; Song, E.; Song, Y. Polychlorinated biphenyl quinone promotes macrophage polarization to CD163(+) cells through Nrf2 signaling pathway. Environ Pollut 2020, 257, 113587.
- Chen, X.; Su, L.; Yang, Y.; Qv, J.; Wei, T.; Cui, X.; Shao, J.; Liu, S.; Xu, Z.; Zhao, B.; Miao, J. A new activator of esterase D decreases blood cholesterol level through ESD/JAB1/ABCA1 pathway. J Cell Physiol 2021, 236, 4750-63.
- Hupfeld, T.; Chapuy, B.; Schrader, V.; Beutler, M.; Veltkamp, C.; Koch, R.; Cameron, S.; Aung, T.; Haase, D.; Larosee, P.; Truemper, L.; Wulf, G. G. Tyrosinekinase inhibition facilitates cooperation of transcription factor SALL4 and ABC transporter A3 towards intrinsic CML cell drug resistance. Br J Haematol 2013, 161, 204-13.
- Sribenja, S.; Natthasirikul, N.; Vaeteewoottacharn, K.; Sawanyawisuth, K.; Wongkham, C.; Jearanaikoon, P.; Wongkham, S. Thymosin beta10 as a predictive biomarker of response to 5-fluorouracil chemotherapy in cholangiocarcinoma. Ann Hepatol 2016, 15, 577-85.
- Quezada, C. A.; Garrido, W. X.; Gonzalez-Oyarzun, M. A.; Rauch, M. C.; Salas, M. R.; San Martin, R. E.; Claude, A. A.; Yanez, A. J.; Slebe, J. C.; Carcamo, J. G. Effect of tacrolimus on activity and expression of P-glycoprotein and ATP-binding cassette transporter A5 (ABCA5) proteins in hematoencephalic barrier cells. Biol Pharm Bull 2008, 31, 1911-6.
- Gai, J.; Ji, M.; Shi, C.; Li, W.; Chen, S.; Wang, Y.; Li, H. FoxO regulates expression of ABCA6, an intracellular ATP-binding-cassette transporter responsive to cholesterol. Int J Biochem Cell Biol 2013, 45, 2651-9.
- Wang, X.; Cao, C.; Li, Y.; Hai, T.; Jia, Q.; Zhang, Y.; Zheng, Q.; Yao, J.; Qin, G.; Zhang, H.; Song, R.; Wang, Y.; Shui, G.; Lam, S. M.; Liu, Z.; Wei, H.; Meng, A.; Zhou, Q.; Zhao, J. A harlequin ichthyosis pig model with a novel ABCA12 mutation can be rescued by acitretin treatment. J Mol Cell Biol 2019, 11, 1029-41.
- Tanaka, N.; Abe-Dohmae, S.; Iwamoto, N.; Fitzgerald, M. L.; Yokoyama, S. HMG-CoA reductase inhibitors enhance phagocytosis by upregulating ATP-binding cassette transporter A7. Atheroscler 2011, 217, 407-14.
- Akisato, Y.; Ishii, I.; Kitahara, M.; Tamaki, T.; Saito, Y.; Kitada, M. [Effect of pitavastatin on macrophage cholesterol metabolism]. Yakugaku Zasshi 2008, 128, 357-63.
- Zhang, Y.; Hu, Z.; Ye, M.; Pan, Y.; Chen, J.; Luo, Y.; Zhang, Y.; He, L.; Wang, J. Effect of poly(ethylene glycol)-block-polylactide nanoparticles on hepatic cells of mouse: low cytotoxicity, but efflux of the nanoparticles by ATP-binding cassette transporters. Eur J Pharm Biopharm 2007, 66, 268-80.
- Jiang, Y. J.; Lu, B.; Kim, P.; Paragh, G.; Schmitz, G.; Elias, P. M.; Feingold, K. R. PPAR and LXR activators regulate ABCA12 expression in human keratinocytes. J Invest Dermatol 2008, 128, 104-9.
- Jiang, Y. J.; Uchida, Y.; Lu, B.; Kim, P.; Mao, C.; Akiyama, M.; Elias, P. M.; Holleran, W. M.; Grunfeld, C.; Feingold, K. R. Ceramide stimulates ABCA12 expression via peroxisome proliferator-activated receptor {delta} in human keratinocytes. J Biol Chem 2009, 284, 18942-52.
- Banno, A.; Wang, J.; Okada, K.; Mori, R.; Mijiti, M.; Nagaoka, S. Identification of a novel cholesterol-lowering dipeptide, phenylalanine-proline (FP), and its down-regulation of intestinal ABCA1 in hypercholesterolemic rats and Caco-2 cells. Sci Rep 2019, 9, 19416.
- Huang, W.; Zhou, J.; Zhang, G.; Zhang, Y.; Wang, H. Decreased H3K9 acetylation level of LXRalpha mediated dexamethasone-induced placental cholesterol transport dysfunction. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 158524.
- Han, Q. A.; Li, K.; Dong, X.; Luo, Y.; Zhu, B. Function of Thelenota ananas saponin desulfated holothurin A in modulating cholesterol metabolism. Sci Rep 2018, 8, 9506.
- Kaplan, M.; Aviram, M.; Knopf, C.; Keidar, S. Angiotensin II reduces macrophage cholesterol efflux: a role for the AT-1 receptor but not for the ABC1 transporter. Biochem Biophys Res Commun 2002, 290, 1529-34.
- Yang, Y.; Yang, Q.; Yang, J.; Ma, Y.; Ding, G. Angiotensin II induces cholesterol accumulation and injury in podocytes. Sci Rep 2017, 7, 10672.
- Boulate, G.; Amazit, L.; Naman, A.; Seck, A.; Paci, A.; Lombes, A.; Pussard, E.; Baudin, E.; Lombes, M.; Hescot, S. Potentiation of mitotane action by rosuvastatin: New insights for adrenocortical carcinoma management. Int J Oncol 2019, 54, 2149-56.
- Wu, X.; Li, C.; Mariyam, Z.; Jiang, P.; Zhou, M.; Zeb, F.; Haq, I. U.; Chen, A.; Feng, Q. Acrolein-induced atherogenesis by stimulation of hepatic flavin containing monooxygenase 3 and a protection from hydroxytyrosol. J Cell Physiol 2018, 234, 475-85.
- Samardzija, D.; Pogrmic-Majkic, K.; Fa, S.; Stanic, B.; Jasnic, J.; Andric, N. Bisphenol A decreases progesterone synthesis by disrupting cholesterol homeostasis in rat granulosa cells. Mol Cell Endocrinol 2018, 461, 55-63.
- Kant, R.; Lu, C. K.; Nguyen, H. M.; Hsiao, H. H.; Chen, C. J.; Hsiao, H. P.; Lin, K. J.; Fang, C. C.; Yen, C. H. 1,2,3,4,6 penta-O-galloyl-beta-D-glucose ameliorates high-fat diet-induced nonalcoholic fatty liver disease and maintains the expression of genes involved in lipid homeostasis in mice. Biomed Pharmacother 2020, 129, 110348.
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. Biomed Res Int 2020, 2020, 5036572.
- Li, Y.; Wu, S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol Cell Biochem 2018, 448, 175-85.
- Chen, J. H.; Zheng, Y. L.; Xu, C. Q.; Gu, L. Z.; Ding, Z. L.; Qin, L.; Wang, Y.; Fu, R.; Wan, Y. F.; Hu, C. P. Valproic acid (VPA) enhances cisplatin sensitivity of non-small cell lung cancer cells via HDAC2 mediated down regulation of ABCA1. Biol Chem 2017, 398, 785-92.
- Koga, M.; Kanaoka, Y.; Okamoto, M.; Nakao, Y.; Inada, K.; Takayama, S.; Kataoka, Y.; Yamauchi, A. Varenicline aggravates atherosclerotic plaque formation in nicotine-pretreated ApoE knockout mice due to enhanced oxLDL uptake by macrophages through downregulation of ABCA1 and ABCG1 expression. J Pharmacol Sci 2020, 142, 9-15.
- Aung, T.; Chapuy, B.; Vogel, D.; Wenzel, D.; Oppermann, M.; Lahmann, M.; Weinhage, T.; Menck, K.; Hupfeld, T.; Koch, R.; Trumper, L.; Wulf, G. G. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A 2011, 108, 15336-41.
- Song, J. H.; Kim, S. H.; Kim, H. J.; Hwang, S. Y.; Kim, T. S. Alleviation of the drug-resistant phenotype in idarubicin and cytosine arabinoside double-resistant acute myeloid leukemia cells by indomethacin. Int J Oncol 2008, 32, 931-6.
- Dai, W.; Wang, F.; He, L.; Lin, C.; Wu, S.; Chen, P.; Zhang, Y.; Shen, M.; Wu, D.; Wang, C.; Lu, J.; Zhou, Y.; Xu, X.; Xu, L.; Guo, C. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: partial mediation by the transcription factor NFAT1. Mol Carcinog 2015, 54, 301-11.
- Long, Y.; Wang, G.; Li, K.; Zhang, Z.; Zhang, P.; Zhang, J.; Zhang, X.; Bao, Y.; Yang, X.; Wang, P. Oxidative stress and NF-kappaB signaling are involved in LPS induced pulmonary dysplasia in chick embryos. Cell Cycle 2018, 17, 1757-71.
- Mendonca-Torres, M. C.; Roberts, S. S. The translocator protein (TSPO) ligand PK11195 induces apoptosis and cell cycle arrest and sensitizes to chemotherapy treatment in pre- and post-relapse neuroblastoma cell lines. Cancer Biol Ther 2013, 14, 319-26.
- Wakaumi, M.; Ishibashi, K.; Ando, H.; Kasanuki, H.; Tsuruoka, S. Acute digoxin loading reduces ABCA8A mRNA expression in the mouse liver. Clin Exp Pharmacol Physio 2005, 32, 1034-41.
- Kinting, S.; Hoppner, S.; Schindlbeck, U.; Forstner, M. E.; Harfst, J.; Wittmann, T.; Griese, M. Functional rescue of misfolding ABCA3 mutations by small molecular correctors. Hum Mol Genet 2018, 27, 943-53.
- Kinting, S.; Li, Y.; Forstner, M.; Delhommel, F.; Sattler, M.; Griese, M. Potentiation of ABCA3 lipid transport function by ivacaftor and genistein. J Cell Mol Med 2019, 23, 5225-34.
- Liu, Q.; Sabirzhanova, I.; Bergbower, E. A. S.; Yanda, M.; Guggino, W. G.; Cebotaru, L. The CFTR Corrector, VX-809 (Lumacaftor), Rescues ABCA4 Trafficking Mutants: a Potential Treatment for Stargardt Disease. Cell Physiol Biochem 2019, 53, 400-12.
- Sabirzhanova, I.; Lopes Pacheco, M.; Rapino, D.; Grover, R.; Handa, J. T.; Guggino, W. B.; Cebotaru, L. rescuing trafficking mutants of the ATP-binding cassette protein, ABCA4, with small molecule correctors as a treatment for stargardt eye disease. J Biol Chem 2015, 290, 19743-55.
- Lopes-Pacheco, M.; Sabirzhanova, I.; Rapino, D.; Morales, M. M.; Guggino, W. B.; Cebotaru, L. correctors rescue CFTR mutations in nucleotide-binding domain 1 (NBD1) by modulating proteostasis. Chembiochem 2016, 17, 493-505.
- Wang, Y.; Loo, T. W.; Bartlett, M. C.; Clarke, D. M. Correctors promote maturation of cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by binding to the protein. J Biol Chem 2007, 282, 33247-51.
- Lin, S.; Zhou, C.; Neufeld, E.; Wang, Y. H.; Xu, S. W.; Lu, L.; Wang, Y.; Liu, Z. P.; Li, D.; Li, C.; Chen, S.; Le, K.; Huang, H.; Liu, P.; Moss, J.; Vaughan, M.; Shen, X. BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein modulates ATP-binding cassette transporter A-1 trafficking and function. Arterioscler Thromb Vasc Biol 2013, 33, e31-8.
- Remaley, A. T.; Schumacher, U. K.; Stonik, J. A.; Farsi, B. D.; Nazih, H.; Brewer, H. B., Jr. Decreased reverse cholesterol transport from Tangier disease fibroblasts. Acceptor specificity and effect of brefeldin on lipid efflux. Arterioscler Thromb Vasc Biol 1997, 17, 1813-21.
- Mendez, A. J. Monensin and brefeldin A inhibit high density lipoprotein-mediated cholesterol efflux from cholesterol-enriched cells. Implications for intracellular cholesterol transport. J Biol Chem 1995, 270, 5891-900.
- Neufeld, E. B.; Remaley, A. T.; Demosky, S. J.; Stonik, J. A.; Cooney, A. M.; Comly, M.; Dwyer, N. K.; Zhang, M.; Blanchette-Mackie, J.; Santamarina-Fojo, S.; Brewer, H. B., Jr. Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem 2001, 276, 27584-90.
- Field, F. J.; Born, E.; Chen, H.; Murthy, S.; Mathur, S. N. Esterification of plasma membrane cholesterol and triacylglycerol-rich lipoprotein secretion in CaCo-2 cells: possible role of p-glycoprotein. J Lipid Res 1995, 36, 1533-43.
- Castilho, G.; Okuda, L. S.; Pinto, R. S.; Iborra, R. T.; Nakandakare, E. R.; Santos, C. X.; Laurindo, F. R.; Passarelli, M. ER stress is associated with reduced ABCA-1 protein levels in macrophages treated with advanced glycated albumin - reversal by a chemical chaperone. Int J Biochem Cell Biol 2012, 44, 1078-86.
- Singaraja, R. R.; Kang, M. H.; Vaid, K.; Sanders, S. S.; Vilas, G. L.; Arstikaitis, P.; Coutinho, J.; Drisdel, R. C.; El-Husseini Ael, D.; Green, W. N.; Berthiaume, L.; Hayden, M. R. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ Res 2009, 105, 138-47.
- Jones, R. J.; Gu, D.; Bjorklund, C. C.; Kuiatse, I.; Remaley, A. T.; Bashir, T.; Vreys, V.; Orlowski, R. Z. The novel anticancer agent JNJ-26854165 induces cell death through inhibition of cholesterol transport and degradation of ABCA1. J Pharmacol Exp Ther 2013, 346, 381-92.
- Roberts, A. G. The Structure and Mechanism of Drug Transporters. Methods in molecular biology (Clifton, N.J) 2021, 2342, 193-234.
- Qian, H.; Zhao, X.; Cao, P.; Lei, J.; Yan, N.; Gong, X. Structure of the Human Lipid Exporter ABCA1. Cell 2017, 169, 1228-1239 e10.
- Scortecci, J. F.; Molday, L. L.; Curtis, S. B.; Garces, F. A.; Panwar, P.; Van Petegem, F.; Molday, R. S. Cryo-EM structures of the ABCA4 importer reveal mechanisms underlying substrate binding and Stargardt disease. Nat Commun 2021, 12, 5902.
- Liu, F.; Lee, J.; Chen, J. Molecular structures of the eukaryotic retinal importer ABCA4. Elife 2021, 10, e63524.
- Xie, T.; Zhang, Z.; Fang, Q.; Du, B.; Gong, X. Structural basis of substrate recognition and translocation by human ABCA4. Nat Commun 2021, 12, 3853.
- My Le, L. T.; Thompson, J. R.; Aikawa, T.; Kanikeyo, T.; Alam, A. Cryo-EM structure of lipid embedded human ABCA7 at 3.6Å resolution. bioRxiv 2021, 2021.03.01.433448.
- Namasivayam, V. S., K.; Pahnke, J.; Stefan, S. M. . Binding mode analysis of PAN-ABC transporter inhibitors within human ABCA7 to target Alzheimer’s disease. Comput Struct Biotechnol J 2021, 19, 6490-6504.
- Alam, A.; Kowal, J.; Broude, E.; Roninson, I.; Locher, K. P. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 2019, 363, 753-6.
- Fitzgerald, M. L.; Morris, A. L.; Rhee, J. S.; Andersson, L. P.; Mendez, A. J.; Freeman, M. W. Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J Biol Chem 2002, 277, 33178-87.
- Lee, J. Y.; Kinch, L. N.; Borek, D. M.; Wang, J.; Wang, J.; Urbatsch, I. L.; Xie, X. S.; Grishin, N. V.; Cohen, J. C.; Otwinowski, Z.; Hobbs, H. H.; Rosenbaum, D. M. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 2016, 533, 561-4.
- Adzhubei, A. A.; Kulkarni, A.; Tolstova, A. P.; Anashkina, A. A.; Sviridov, D.; Makarov, A. A.; Bukrinsky, M. I. Direct interaction between ABCA1 and HIV-1 Nef: Molecular modeling and virtual screening for inhibitors. Comput Struct Biotechnol J 2021, 19, 3876-84.
- Krapf, M. K.; Gallus, J.; Namasivayam, V.; Wiese, M. 2,4,6-substituted quinazolines with extraordinary inhibitory potency toward ABCG2. J Med Chem 2018, 61, 7952-76.
- Jackson, S. M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N. M. I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; Buschauer, A.; Stahlberg, H.; Altmann, K. H.; Locher, K. P. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol 2018, 25, 333-40.
- Rarey, M.; Kramer, B.; Lengauer, T. Time-efficient docking of flexible ligands into active sites of proteins. Proc Int Conf Intell Syst Mol Biol 1995, 3, 300-8.
- Ni, Z.; Bikadi, Z.; Cai, X.; Rosenberg, M. F.; Mao, Q. Transmembrane helices 1 and 6 of the human breast cancer resistance protein (BCRP/ABCG2): identification of polar residues important for drug transport. Am J Physiol Cell Physiol 2010, 299, C1100-9.
- Tamura, A.; Watanabe, M.; Saito, H.; Nakagawa, H.; Kamachi, T.; Okura, I.; Ishikawa, T. Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: identification of alleles that are defective in porphyrin transport. Mol Pharmacol 2006, 70, 287-96.
- Ozvegy, C.; Varadi, A.; Sarkadi, B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J Biol Chem 2002, 277, 47980-90.
- Volk, E. L.; Farley, K. M.; Wu, Y.; Li, F.; Robey, R. W.; Schneider, E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res 2002, 62, 5035-40.
- Honjo, Y.; Hrycyna, C. A.; Yan, Q. W.; Medina-Perez, W. Y.; Robey, R. W.; van de Laar, A.; Litman, T.; Dean, M.; Bates, S. E. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res 2001, 61, 6635-9.
- Silbermann, K.; Li, J.; Namasivayam, V.; Stefan, S. M.; Wiese, M. Rational drug design of 6-substituted 4-anilino-2-phenylpyrimidines for exploration of novel ABCG2 binding site. Eur J Med Chem 2021, 212, 113045.
- Silbermann, K.; Li, J.; Namasivayam, V.; Baltes, F.; Bendas, G.; Stefan, S. M.; Wiese, M. Superior pyrimidine derivatives as selective ABCG2 inhibitors and broad-spectrum ABCB1, ABCC1, and ABCG2 Antagonists. J Med Chem 2020, 63, 10412-32.
- Kim, S.; Thiessen, P. A.; Bolton, E. E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B. A.; Wang, J.; Yu, B.; Zhang, J.; Bryant, S. H. PubChem substance and compound databases. Nucleic Acids Res 2016, 44, D1202-13.
- Rogers, D.; Hahn, M. Extended-connectivity fingerprints. J Chem Inf Model 2010, 50, 742-54.
- Bender, A.; Mussa, H. Y.; Glen, R. C.; Reiling, S. Molecular similarity searching using atom environments, information-based feature selection, and a naive Bayesian classifier. J Chem Inf Comput Sci 2004, 44, 170-8.
- Silbermann, K.; Stefan, S. M.; Elshawadfy, R.; Namasivayam, V.; Wiese, M. Identification of thienopyrimidine scaffold as an inhibitor of the abc transport protein ABCC1 (MRP1) and related transporters using a combined virtual screening approach. J Med Chem 2019, 62, 4383-400.
- Danish, A.; Namasivayam, V.; Schiedel, A. C.; Muller, C. E. Interaction of approved drugs with synaptic vesicle protein 2A. Arch Pharm (Weinheim) 2017, 350.
- Namasivayam, V. S., K.; Pahnke, J.; Wiese, M.; Stefan, S. M. Feature-driven pattern analysis for multitarget modulator landscapes. Bioinformatics 2021, https://www.doi.org/10.1093/bioinformatics/btab832
- Lee, Y. M.; Venkataraman, K.; Hwang, S. I.; Han, D. K.; Hla, T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC). Prostaglandins Other Lipid Mediat 2007, 84, 154-62.
- Oram, J. F.; Vaughan, A. M.; Stocker, R. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J Biol Chem 2001, 276, 39898-902.
- Haller, J. F.; Cavallaro, P.; Hernandez, N. J.; Dolat, L.; Soscia, S. J.; Welti, R.; Grabowski, G. A.; Fitzgerald, M. L.; Freeman, M. W. Endogenous beta-glucocerebrosidase activity in Abca12(-)/(-)epidermis elevates ceramide levels after topical lipid application but does not restore barrier function. J Lipid Res 2014, 55, 493-503.
- Reboul, E.; Dyka, F. M.; Quazi, F.; Molday, R. S. Cholesterol transport via ABCA1: new insights from solid-phase binding assay. Biochim 2013, 95, 957-61.
- Beljanski, V.; Soulika, A.; Tucker, J. M.; Townsend, D. M.; Davis, W., Jr.; Tew, K. D. Characterization of the ATPase activity of human ATP-binding cassette transporter-2 (ABCA2). In vivo (Athens, Greece) 2005, 19, 657-60.
- Tsybovsky, Y.; Wang, B.; Quazi, F.; Molday, R. S.; Palczewski, K. Posttranslational modifications of the photoreceptor-specific ABC transporter ABCA4. Biochem 2011, 50, 6855-66.
- Zhong, M.; Molday, L. L.; Molday, R. S. Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function, and retinal degenerative diseases. J Biol Chem 2009, 284, 3640-9.
- Petry, F.; Ritz, V.; Meineke, C.; Middel, P.; Kietzmann, T.; Schmitz-Salue, C.; Hirsch-Ernst, K. I. Subcellular localization of rat Abca5, a rat ATP-binding-cassette transporter expressed in Leydig cells, and characterization of its splice variant apparently encoding a half-transporter. Biochem J 2006, 393, 79-87.
- Hu, J. Y.; Yang, P.; Wegner, D. J.; Heins, H. B.; Luke, C. J.; Li, F.; White, F. V.; Silverman, G. A.; Sessions Cole, F.; Wambach, J. A. Functional characterization of four ATP-binding cassette transporter A3 gene (ABCA3) variants. Hum Mutat 2020, 41, 1298-1307.
- Trompier, D.; Alibert, M.; Davanture, S.; Hamon, Y.; Pierres, M.; Chimini, G. Transition from dimers to higher oligomeric forms occurs during the ATPase cycle of the ABCA1 transporter. J Biol Chem 2006, 281, 20283-90.
- Takahashi, K.; Kimura, Y.; Kioka, N.; Matsuo, M.; Ueda, K. Purification and ATPase activity of human ABCA1. J Biol Chem 2006, 281, 10760-8.
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M. L.; Remiao, F. Cellular models and in vitro assays for the screening of modulators of P-GP, MRP and BCRP. Molecules 2017, 22.
- Kraege, S.; Stefan, K.; Kohler, S. C.; Wiese, M. Optimization of acryloylphenylcarboxamides as inhibitors of ABCG2 and comparison with acryloylphenylcarboxylates. ChemMedChem 2016, 11, 2547-58.
- Spindler, A.; Stefan, K.; Wiese, M. Synthesis and investigation of tetrahydro-beta-carboline derivatives as inhibitors of the breast cancer resistance protein (ABCG2). J Med Chem 2016, 59, 6121-35.
- Kraege, S.; Stefan, K.; Juvale, K.; Ross, T.; Willmes, T.; Wiese, M. The combination of quinazoline and chalcone moieties leads to novel potent heterodimeric modulators of breast cancer resistance protein (BCRP/ABCG2). Eur J Med Chem 2016, 117, 212-29.
- Gelissen, I. C.; Brown, A. J. Methods Mol Biol 2017, 1583, 1–6.
- Yang, A.; Gelissen, I. C. ABC-transporter mediated sterol export from cells using radiolabeled sterols. Methods in molecular biology (Clifton, N.J) 2017, 1583, 275-85.
- Cattelotte, J.; Andre, P.; Ouellet, M.; Bourasset, F.; Scherrmann, J. M.; Cisternino, S. In situ mouse carotid perfusion model: glucose and cholesterol transport in the eye and brain. J Cereb Blood Flow Metab 2008, 28, 1449-59.
- Li, Y.; Kinting, S.; Hoppner, S.; Forstner, M. E.; Uhl, O.; Koletzko, B.; Griese, M. Metabolic labelling of choline phospholipids probes ABCA3 transport in lamellar bodies. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 158516.
- Hoppner, S.; Kinting, S.; Torrano, A. A.; Schindlbeck, U.; Brauchle, C.; Zarbock, R.; Wittmann, T.; Griese, M. Quantification of volume and lipid filling of intracellular vesicles carrying the ABCA3 transporter. Biochim Biophys Acta Mol Cell Res 2017, 1864, 2330-5.
- Cox, J. V.; Abdelrahman, Y. M.; Peters, J.; Naher, N.; Belland, R. J. Chlamydia trachomatis utilizes the mammalian CLA1 lipid transporter to acquire host phosphatidylcholine essential for growth. Cell Microbiol 2016, 18, 305-18.
- Sankaranarayanan, S.; Kellner-Weibel, G.; de la Llera-Moya, M.; Phillips, M. C.; Asztalos, B. F.; Bittman, R.; Rothblat, G. H. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res 2011, 52, 2332-40.
- Stearns, M. E.; Jenkins, D. P.; Tew, K. D. Dansylated estramustine, a fluorescent probe for studies of estramustine uptake and identification of intracellular targets. Proc Natl Acad Sci U S A 1985, 82, 8483-7.
- Bryan, A.; Watters, C.; Koenig, L.; Youn, E.; Olmos, A.; Li, G.; Williams, S. C.; Rumbaugh, K. P. Human transcriptome analysis reveals a potential role for active transport in the metabolism of Pseudomonas aeruginosa autoinducers. Microbes Infect 2010, 12, 1042-50.
- Chaudhuri, A.; Anand, D. Cholesterol: Revisiting its fluorescent journey on 200th anniversary of Chevruel’s “cholesterine”. Biomed Spectrosc Imaging 2017, 6, 1-24.
- Solanko, K. A.; Modzel, M.; Solanko, L. M.; Wustner, D. Fluorescent sterols and cholesteryl esters as probes for intracellular cholesterol transport. Lipid Insights 2015, 8, 95-114.
- Invitrogen. Chapter 13 - Probes for lipids and membranes. In Molecular probes handbook: a guide to fluorescent probes and labeling technologies, ThermoFischer, Ed. 2010.
- Silbermann, K.; Shah, C. P.; Sahu, N. U.; Juvale, K.; Stefan, S. M.; Kharkar, P. S.; Wiese, M. Novel chalcone and flavone derivatives as selective and dual inhibitors of the transport proteins ABCB1 and ABCG2. Eur J Med Chem 2019, 164, 193-213.
- Stefan, K.; Schmitt, S. M.; Wiese, M. 9-Deazapurines as broad-spectrum inhibitors of the ABC transport proteins P-glycoprotein, multidrug resistance-associated protein 1, and breast cancer resistance protein. J Med Chem 2017, 60, 8758-80.
- Schmitt, S. M.; Stefan, K.; Wiese, M. Pyrrolopyrimidine derivatives as novel inhibitors of multidrug resistance-associated protein 1 (MRP1, ABCC1). J Med Chem 2016, 59, 3018-33.
- Stefan, K. Etablierung und Anwendung unterschiedlicher kolorimetrischer Detektionsmethoden zur Aktivitätsbestimmung von Modulatoren der ABC-Transporter ABCB1, ABCC1 und ABCG2. Rheinische Friedrich-Wilhelms-Universität Bonn, 2020.
- Mack, J. T.; Beljanski, V.; Soulika, A. M.; Townsend, D. M.; Brown, C. B.; Davis, W.; Tew, K. D. "Skittish" ABCA2 knockout mice display tremor, hyperactivity, and abnormal myelin ultrastructure in the central nervous system. Mol Cell Biol 2007, 27, 44-53.
- Ban, N.; Matsumura, Y.; Sakai, H.; Takanezawa, Y.; Sasaki, M.; Arai, H.; Inagaki, N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem 2007, 282, 9628-34.
- Hammel, M.; Michel, G.; Hoefer, C.; Klaften, M.; Müller-Höcker, J.; de Angelis, M. H.; Holzinger, A. Targeted inactivation of the murine Abca3 gene leads to respiratory failure in newborns with defective lamellar bodies. Biochem Biophys Res Commun 2007, 359, 947-51.
- Beers, M. F.; Knudsen, L.; Tomer, Y.; Maronn, J.; Zhao, M.; Ochs, M.; Mulugeta, S. Aberrant lung remodeling in a mouse model of surfactant dysregulation induced by modulation of the Abca3 gene. Ann Anat 2017, 210, 135-46.
- Weng, J.; Mata, N. L.; Azarian, S. M.; Tzekov, R. T.; Birch, D. G.; Travis, G. H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 1999, 98, 13-23.
- Molday, R. S. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr 2007, 39, 507-17.
- Trigueros-Motos, L.; van Capelleveen, J. C.; Torta, F.; Castaño, D.; Zhang, L. H.; Chai, E. C.; Kang, M.; Dimova, L. G.; Schimmel, A. W. M.; Tietjen, I.; Radomski, C.; Tan, L. J.; Thiam, C. H.; Narayanaswamy, P.; Wu, D. H.; Dorninger, F.; Yakala, G. K.; Barhdadi, A.; Angeli, V.; Dubé, M. P.; Berger, J.; Dallinga-Thie, G. M.; Tietge, U. J. F.; Wenk, M. R.; Hayden, M. R.; Hovingh, G. K.; Singaraja, R. R. ABCA8 regulates cholesterol efflux and high-density lipoprotein cholesterol levels. Arterioscler Thromb Vasc Biol 2017, 37, 2147-55.
- Iritani, S.; Torii, Y.; Habuchi, C.; Sekiguchi, H.; Fujishiro, H.; Yoshida, M.; Go, Y.; Iriki, A.; Isoda, M.; Ozaki, N. The neuropathological investigation of the brain in a monkey model of autism spectrum disorder with ABCA13 deletion. Int J Dev Neurosci 2018, 71, 130-9.
- LePage, D. F.; Conlon, R. A. Animal models for disease: knockout, knock-in, and conditional mutant mice. Methods Mol Med 2006, 129, 41-67.
- Navabpour, S.; Kwapis, J. L.; Jarome, T. J. A neuroscientist's guide to transgenic mice and other genetic tools. Neurosci Biobehav Rev 2020, 108, 732-48.
- Campenhout, C. V.; Cabochette, P.; Veillard, A. C.; Laczik, M.; Zelisko-Schmidt, A.; Sabatel, C.; Dhainaut, M.; Vanhollebeke, B.; Gueydan, C.; Kruys, V. Guidelines for optimized gene knockout using CRISPR/Cas9. Biotechniques 2019, 66, 295-302.
- Lovett-Racke, A. E.; Cravens, P. D.; Gocke, A. R.; Racke, M. K.; Stüve, O. Therapeutic potential of small interfering RNA for central nervous system diseases. Arch Neurol 2005, 62, 1810-3.
- Moore, C. B.; Guthrie, E. H.; Huang, M. T.; Taxman, D. J. Short hairpin RNA (shRNA): design, delivery, and assessment of gene knockdown. Methods Mol Biol (Clifton, N.J) 2010, 629, 141-58.
- Drummond, E.; Wisniewski, T. Alzheimer's disease: experimental models and reality. Acta Neuropathol 2017, 133, 155-75.
- Mochizuki, H.; Yamada, M.; Mizuno, Y. Alpha-synuclein overexpression model. J Neural Transm 2006, 281-4.
- Peltz, G. Can 'humanized' mice improve drug development in the 21st century? Trends Pharmacol Sci 2013, 34, 255-60.
- Krohn, M.; Zoufal, V.; Mairinger, S.; Wanek, T.; Paarmann, K.; Brüning, T.; Eiriz, I.; Brackhan, M.; Langer, O.; Pahnke, J. Generation and characterization of an abcc1 humanized mouse model (HABCC1(FLX/FLX)) with knockout capability. Mol Pharmacol 2019, 96, 138-147.
- Dallas, S.; Salphati, L.; Gomez-Zepeda, D.; Wanek, T.; Chen, L.; Chu, X.; Kunta, J.; Mezler, M.; Menet, M. C.; Chasseigneaux, S.; Decleves, X.; Langer, O.; Pierre, E.; DiLoreto, K.; Hoft, C.; Laplanche, L.; Pang, J.; Pereira, T.; Andonian, C.; Simic, D.; Rode, A.; Yabut, J.; Zhang, X.; Scheer, N. generation and characterization of a breast cancer resistance protein humanized mouse model. Mol Pharmacol 2016, 89, 492-504.
- Krohn, M.; Wanek, T.; Menet, M. C.; Noack, A.; Decleves, X.; Langer, O.; Loscher, W.; Pahnke, J. Humanization of the blood-brain barrier transporter ABCB1 in mice disrupts genomic locus - lessons from three unsuccessful approaches. Eur J Microbiol Immunol (Bp) 2018, 8, 78-86.
- Willmann, J. K.; van Bruggen, N.; Dinkelborg, L. M.; Gambhir, S. S. Molecular imaging in drug development. Nat Rev Drug Discov 2008, 7, 591-607.
- Mairinger, S.; Erker, T.; Muller, M.; Langer, O. PET and SPECT radiotracers to assess function and expression of ABC transporters in vivo. Curr Drug Metab 2011, 12, 774-92.
- Zoufal, V.; Mairinger, S.; Krohn, M.; Wanek, T.; Filip, T.; Sauberer, M.; Stanek, J.; Traxl, A.; Schuetz, J. D.; Kuntner, C.; Pahnke, J.; Langer, O. Influence of multidrug resistance-associated proteins on the excretion of the ABCC1 imaging probe 6-bromo-7-[(11)C]methylpurine in mice. Mol Imaging Biol 2019, 21, 306-16.
- Wanek, T.; Zoufal, V.; Brackhan, M.; Krohn, M.; Mairinger, S.; Filip, T.; Sauberer, M.; Stanek, J.; Pekar, T.; Pahnke, J.; Langer, O. Brain Distribution of dual ABCB1/ABGC2 substrates is unaltered in a beta-amyloidosis mouse model. Int J Mol Sci 2020, 21, 8245.
- Zoufal, V.; Wanek, T.; Krohn, M.; Mairinger, S.; Filip, T.; Sauberer, M.; Stanek, J.; Pekar, T.; Bauer, M.; Pahnke, J.; Langer, O. Age dependency of cerebral P-glycoprotein function in wild-type and APPPS1 mice measured with PET. J Cereb Blood Flow Metab 2018, 40, 150-62.
- Campbell, B. R.; Gonzalez Trotter, D.; Hines, C. D.; Li, W.; Patel, M.; Zhang, W.; Evelhoch, J. L. In vivo imaging in pharmaceutical development and its impact on the 3Rs. Ilar j 2016, 57, 212-20.
- Bieczynski, F.; Burkhardt-Medicke, K.; Luquet, C. M.; Scholz, S.; Luckenbach, T. Chemical effects on dye efflux activity in live zebrafish embryos and on zebrafish Abcb4 ATPase activity. FEBS Lett 2021, 595, 828-43.
- Pedersen, J. M.; Matsson, P.; Bergstrom, C. A.; Hoogstraate, J.; Noren, A.; LeCluyse, E. L.; Artursson, P. Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci 2013, 136, 328-43.
- Matsson, P.; Pedersen, J. M.; Norinder, U.; Bergstrom, C. A.; Artursson, P. Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm Res 2009, 26, 1816-31.
- Cserepes, J.; Szentpetery, Z.; Seres, L.; Ozvegy-Laczka, C.; Langmann, T.; Schmitz, G.; Glavinas, H.; Klein, I.; Homolya, L.; Varadi, A.; Sarkadi, B.; Elkind, N. B. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: indications for heterodimerization. Biochem Biophys Res Commun 2004, 320, 860-7.
- Ivnitski-Steele, I.; Larson, R. S.; Lovato, D. M.; Khawaja, H. M.; Winter, S. S.; Oprea, T. I.; Sklar, L. A.; Edwards, B. S. High-throughput flow cytometry to detect selective inhibitors of ABCB1, ABCC1, and ABCG2 transporters. Assay Drug Dev Technol 2008, 6, 263-76.
- Borst, P.; de Wolf, C.; van de Wetering, K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch 2007, 453, 661-73.
- Berghaus, A.; Jovanovic, S. Technique and indications of extended sublabial rhinotomy ("midfacial degloving"). Rhinol 1991, 29, 105-10.
- Lim, J. G.; Lee, H. Y.; Yun, J. E.; Kim, S. P.; Park, J. W.; Suh, S. I.; Jang, B. C.; Cho, C. H.; Bae, J. H.; Kim, S. S.; Han, J.; Park, M. J.; Song, D. K. Taurine block of cloned ATP-sensitive K+ channels with different sulfonylurea receptor subunits expressed in Xenopus laevis oocytes. Biochem Pharmacol 2004, 68, 901-10.
- York, N. W.; Parker, H.; Xie, Z.; Tyus, D.; Waheed, M. A.; Yan, Z.; Grange, D. K.; Remedi, M. S.; England, S. K.; Hu, H.; Nichols, C. G. Kir6.1- and SUR2-dependent KATP over-activity disrupts intestinal motility in murine models of Cantu Syndrome. JCI Insight 2020, 5, e141443.
- Beretta, G. L.; Cassinelli, G.; Pennati, M.; Zuco, V.; Gatti, L. Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur J Med Chem 2017, 142, 271-89.
- Horikawa, M.; Kato, Y.; Sugiyama, Y. Reduced gastrointestinal toxicity following inhibition of the biliary excretion of irinotecan and its metabolites by probenecid in rats. Pharm Res 2002, 19, 1345-53.
- Smeets, P. H.; van Aubel, R. A.; Wouterse, A. C.; van den Heuvel, J. J.; Russel, F. G. Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J Am Soc Nephrol 2004, 15, 2828-35.
- Dalpiaz, A.; Pavan, B. Nose-to-Brain Delivery of Antiviral Drugs: A Way to Overcome Their Active Efflux? Pharm 2018, 10, 39.
- Zhou, Y.; Hopper-Borge, E.; Shen, T.; Huang, X. C.; Shi, Z.; Kuang, Y. H.; Furukawa, T.; Akiyama, S.; Peng, X. X.; Ashby, C. R., Jr.; Chen, X.; Kruh, G. D.; Chen, Z. S. Cepharanthine is a potent reversal agent for MRP7(ABCC10)-mediated multidrug resistance. Biochem Pharmacol 2009, 77, 993-1001.
- Saeed, M. E. M.; Boulos, J. C.; Elhaboub, G.; Rigano, D.; Saab, A.; Loizzo, M. R.; Hassan, L. E. A.; Sugimoto, Y.; Piacente, S.; Tundis, R.; Yagi, S.; Khalid, H.; Efferth, T. Cytotoxicity of cucurbitacin E from Citrullus colocynthis against multidrug-resistant cancer cells. Phytomedicine 2019, 62, 152945.
- Horikawa, M.; Kato, Y.; Tyson, C. A.; Sugiyama, Y. Potential cholestatic activity of various therapeutic agents assessed by bile canalicular membrane vesicles isolated from rats and humans. Drug Metab Pharmacokinet 2003, 18, 16-22.
- Bai, J.; Lai, L.; Yeo, H. C.; Goh, B. C.; Tan, T. M. Multidrug resistance protein 4 (MRP4/ABCC4) mediates efflux of bimane-glutathione. Int J Biochem Cell Biol 2004, 36, 247-57.
- Videmann, B.; Mazallon, M.; Prouillac, C.; Delaforge, M.; Lecoeur, S. ABCC1, ABCC2 and ABCC3 are implicated in the transepithelial transport of the myco-estrogen zearalenone and its major metabolites. Toxicol Lett 2009, 190, 215-23.
- Tun-Yhong, W.; Chinpaisal, C.; Pamonsinlapatham, P.; Kaewkitichai, S. Tenofovir disoproxil fumarate is a new substrate of ATP-binding cassette subfamily C member 11. Antimicrob Agents Chemother 2017, 61, e01725-16.
Copyright: © 2021 The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited, a link to the Creative Commons license is provided, and any changes are indicated. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
|