|
Contents
Neuroinflammation
NI-01
NI-02
NI-03
Neurooncology Adult I (from Mechanisms to Translation)
NOA-01
NOA-02
NOA-03
NOA-04
NOA-05
NOA-06
NOA-07
Muscle & Nerve Diseases
MND-01
MND-02
Neurodegeneration / COVID-19 and Neuropathology
ND-01
ND-02
ND-03
Neurooncology Pediatrics
NOP-01
NOP-02
Postersession I – Neuroinflammation
PI-01
PI-02
PI-03
Postersession II – Neurooncology
PII-01
PII-02
PII-03
Poster session III – Muscle & Nerve
PIII-01
PIII-02
PIII-03
PIII-04
Poster session IV – Neurodegeneration
PIV-01
PIV-02
PIV-03
PIV-04
PIV-05
PIV-06
Neuroinflammation
NI-01
Free Neuropathol 2:22:4
Microbiota-derived acetate enables the metabolic fitness of the CNS innate immune system during health and disease
D. Erny1
1 Institut für Neuropathologie, Neurozentrum, Freiburg i. Br., Germany
Introduction: As tissue macrophages of the central nervous system (CNS) microglia constitute the pivotal immune cells of this organ. Microglial features are strongly dependent on environmental cues such as commensal microbiota. Gut bacteria are known to continuously modulate microglia maturation and function by the production of short-chain fatty acids (SCFA). However, the precise mechanism of this crosstalk is unknown.
Objectives & Results: Here we determined that the immature phenotype of microglia from germ-free (GF) mice is epigenetically imprinted by H3K4me3 and H3K9ac on metabolic genes associated with substantial functional alterations including increased mitochondrial mass and specific respiratory chain dysfunctions. We identified acetate as the essential microbiome-derived SCFA driving microglia maturation and regulating the homeostatic metabolic state, and further showed that it is able to modulate microglial phagocytosis and disease progression during neurodegeneration.
Conclusion: These findings indicate that acetate is an essential bacteria-derived molecule driving metabolic pathways and functions of microglia during health and perturbation.
NI-02
Free Neuropathol 2:22:5
Multiple sclerosis immunopathological patterns and their MRI correlates
I. Metz1, R. Gavrilova2, S. Weigand3, Y. Guo2, N. Zalewski2, M. Gloth1, O. Tobin2, H. Lassmann4, J. Tillema2, B. Erickson2, J. Parisi5, B. Popescu6, S. Becker7, F. König8, J. Frischer9, W. Brück1, C. Lucchinetti2
1 University Medical Center Göttingen, Georg-August-University, Institute of Neuropathology, Göttingen, Germany
2 Mayo Clinic, Department of Neurology, Rochester, NY, United States
3 Mayo Clinic, Department of Health Sciences Research, Rochester, NY, United States
4 Medical University Vienna, Department of Neuroimmunology, Center for Brain Research, Vienna, Austria
5 Mayo Clinic, Department of Laboratory Medicine and Pathology, Rochester, NY, United States
6 University of Saskatchewan, Department of Anatomy, Physiology and Pharmacology, and Cameco MS Neuroscience Research Center, Saskatchewan, Canada
7 University Medical Center Göttingen, Department of Palliative Medicine, Göttingen, Germany
8 Klinikum Kassel, Institute of Pathology, Kassel, Germany
9 Medical University Vienna, Department of Neurosurgery, Vienna, Austria
Introduction: Early active multiple sclerosis lesions can be classified into three main immunopathological patterns of active demyelination (patterns I-III) by histology. These patterns are inter-individually heterogeneous but intra-individually stable. In pattern I and II, a T-cell- and macrophage-associated demyelination is suggested, with pattern II only showing signs of a humoral immune response. Pattern III is characterized by inflammatory lesions with an oligodendrocyte degeneration. Patterns suggest pathogenic heterogeneity, and we postulated that they have distinct MRI correlates that may serve as biomarkers.
Objectives: To analyze the MRI correlates of multiple sclerosis immunopathological patterns.
Patients and Methods: We evaluated in an international collaborative retrospective cohort study the MRI lesion characteristics of 789 pre-biopsy and follow-up MRIs in relation to their histopathologically classified immunpathological patterns (n=161 subjects) and lesion edge features (n=112).
Results: A strong association of a ring-like enhancement and a hypointense T2-weighted rim (T2w rim) with pattern I and II, but not pattern III, was observed. Only a fraction of pattern III patients showed a ring-like enhancement, and this was always atypical. Ring-like enhancement and T2w rims colocalized, and ring-like enhancement showed a strong association with macrophage rims as shown by histology. A strong concordance of MRI lesion characteristics, meaning that different lesions showed the same features, was found, indicating lesion homogeneity within individual patients.
Conclusion: We provide robust evidence that MRI characteristics reflect specific histological features of MS immunopatterns and that ring-like enhancement and T2w hypointense rims might serve as a valuable non-invasive biomarker to differentiate pathological patterns of demyelination.
NI-03
Free Neuropathol 2:22:7
Microvascular vulnerability to cytokine toxicity is a critical initiating factor in cerebral interferonopathies
B. Viengkhou1, E. Hayashida1, S. McGlasson2, K. Emelianova2, Y. Crow2, A. Pagenstecher3, D. Hunt2, M. Hofer1
1 The University of Sydney, Charles Perkins Centre, Sydney, Australia
2 The University of Edinburgh, Edinburgh, United Kingdom
3 University of Marburg, Dept. Neuropathology, Marburg, Germany
Cerebral interferonopathies are a group of devastating diseases characterised by overproduction of interferon-alpha (IFN-α), leading to diffuse brain damage. Importantly, the critical cellular mediators of IFN-α are unknown.
Here we use a mouse model of brain-targeted IFN-α production to identify endothelial cells as the major mediator of IFN-α neurotoxicity. Specifically, we have created a single cell atlas of the "cerebral interferome" using single cell RNA sequencing of brain cells of wild type and IFN-α transgenic mice. By linking this dataset with neuropathological studies of cerebral interferon overproduction, we identified a role for cerebral endothelial cells in the initiation of brain disease. We then confirmed a critical role for endothelial interferon signalling through targeted deletion of the type I interferon receptor IFNAR on endothelial cells of the transgenic mice. Remarkably, this intervention led to near-complete rescue of the complex multicellular disease phenotype. Importantly, we show that diffuse aspects of interferonopathic brain disease such as calcification and neuronal loss arise as a downstream consequence of cytokine-induced microvascular disease.
In conclusion, our data identify damage to the cerebral microvasculature as the critical first step in interferon neurotoxicity, and pinpoint IFNAR signalling within endothelial cells as a key site for potential therapeutic intervention.
Neurooncology Adult I (from Mechanisms to Translation)
NOA-01
Free Neuropathol 2:22:8
Molecular mechanism of therapy resistance in malignant melanoma brain metastasis
E. Schumann1, R. Koll1, J. Onken2, K. Jürchott3, T. Redmer4, J. Radke1
1 Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
2 Charité – Universitätsmedizin Berlin, Department of Neurosurgery, Berlin, Germany
3 Charité – Universitätsmedizin Berlin, Institute of Medical Immunology, Berlin, Germany
4 University of Veterinary Medicine Vienna, Department of Medical Biochemistry, Vienna, Austria
Introduction: Malignant melanoma (MM) has the highest potential to disseminate to the CNS. About 45% of patients suffer from brain metastasis, which likely proceed continuously during the course of disease. Genetically and molecularly distinct subclones lead to tumor heterogeneity, therapy resistance and poor prognosis. Previous studies suggested increased metastatic spread to and within the brain during BRAF inhibitor (BRAFi) therapy, which is caused by upregulation of a subset of molecular drivers that control migratory and invasion such as the nerve growth factor receptor CD271/NGFR.
Objectives: To investigate the molecular features of migration and invasion of patient derived cell lines from MM brain metastases (BM) that were therapy-responsive or therapy-resistant to BRAFi, radiotherapy and immune checkpoint inhibitors.
Patients & Methods: We isolated and cultured patient derived cell lines from MM BM (n = 9) and DNA-sequencing (n = 5) and transcriptome analyses (n = 2) of cell lines and concordant tumor (n = 2). The Incucyte® Live-Cell Analysis was used to perform high throughput scratch wound assays with patient derived cell lines, which were genetically modified to overexpress or downregulate the expression of NGFR.
Results: Transcriptome profiling of BRAFi resistant MM BM revealed that the invasive potential increased during disease progression, which was accompanied by upregulation of NGFR expression. This phenotype was preserved in patient derived cells lines, which demonstrated significantly higher potential of two-dimensional in vitro migration (90% vs. 76% after 100 hours). Furthermore, CD271 knockdown was associated with loss-of-expression of several genes involved in migration and invasion such as ERBB3, TCF19 and BAALC.
Conclusion: Brain metastases are the major cause of death in metastasized MM. Our study provides a longitudinal perspective on the progression of brain metastasis and their mechanisms leading to therapy resistance.
NOA-02
Free Neuropathol 2:22:9
Molecular characterization of sporadic endolymphatic sac tumours and comparison to von Hippel-Lindau disease-related tumours
L. Schweizer1, F. Thierfelder1, C. Thomas2, P. Soschinski2, H. Y. Kim1, R. Jödicke1, N. Woltering2, A. Förster1, D. Teichmann1, C. Siewert1, K. Klein1, S. Schmid1, M. Nunninger3, U. W. Thomale4, J. Onken5, H. Mühleisen6, J. Schittenhelm7, M. Tatagiba8, A. von Deimling9, D. E. Reuss9, D. A. Solomon10, F. L. Heppner1, A. Koch1, C. Hartmann11, O. Staszewski12, D. Capper1
1 Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
2 University Hospital Münster, Department of Neuropathology, Münster, Germany
3 Charité – Universitätsmedizin Berlin, Department of Radiology, Berlin, Germany
4 Charité – Universitätsmedizin Berlin, Department of Neurosurgery, Division Pediatric Neurosurgery, Berlin, Germany
5 Charité – Universitätsmedizin Berlin, Department of Neurosurgery, Berlin, Germany
6 SYNLAB Labor für Pathologie Mutlangen, Mutlangen, Germany
7 Institute of Pathology and Neuropathology, University of Tübingen, Department of Neuropathology, Tübingen, Germany
8 University Hospital Tübingen, Department of Neurosurgery, Tübingen, Germany
9 Heidelberg University Hospital, Department of Neuropathology, Institute of Pathology, Heidelberg, Germany
10 University of California, Division of Neuropathology, Department of Pathology, San Francisco, CA, United States
11 Hannover Medical School (MHH), Department of Neuropathology, Hannover, Germany
12 Faculty of Medicine, University of Freiburg, Institute of Neuropathology, Freiburg i. Br., Germany
Introduction. Endolymphatic sac tumours (ELSTs) are low-grade tumours arising from the endolymphatic sac in the temporal bone. ELSTs can be locally aggressive and may even expand into the posterior fossa representing a rare differential diagnosis for tumours of the cerebellopontine angle. Although inactivation of the von Hippel-Lindau gene (VHL) on chromosome 3p25 is considered to be the major cause of hereditary endolymphatic sac tumours (ELSTs), the genetic background of sporadic ELST is largely unknown.
Objectives. The aim of this study was to determine the prevalence of VHL alterations in sporadic ELSTs and compare their characteristics to VHL-disease-related tumours.
Materials and Methods. We analyzed 11 sporadic and 11 VHL-disease-related ELSTs by targeted sequencing and DNA methylation analysis.
Results. VHL mutations and small deletions detected by targeted deep sequencing were identified in 9/11 sporadic ELSTs (82%). No other cancer-related genetic pathway was altered except for TERT promoter mutations in two sporadic and one VHL-disease-related ELSTs (15%). Loss of heterozygosity of chromosome 3 was found in 6/10 (60%) VHL-disease-related and 10/11 (91%) sporadic ELSTs resulting in biallelic VHL inactivation in 8/10 (73%) sporadic ELSTs. DNA methylation profiling did not reveal differences between sporadic and VHL-disease-related ELSTs, but reliably distinguished ELST from morphological mimics of the cerebellopontine angle. VHL patients were significantly younger at disease onset compared to sporadic ELSTs (29 vs. 52 years, p < 0.0001, Fisher's exact test). VHL-disease status was not associated with an increased risk of recurrence, but the presence of clear cells was found to be associated with shorter progression-free survival (p = 0.0002, log-rank test).
Conclusion. Biallelic inactivation of VHL is the main mechanism underlying ELSTs, but unknown mechanisms beyond VHL may rarely be involved in the pathogenesis of sporadic ELSTs.
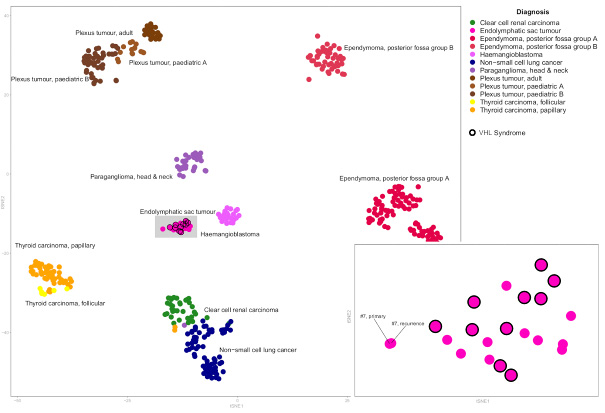
Fig. 1
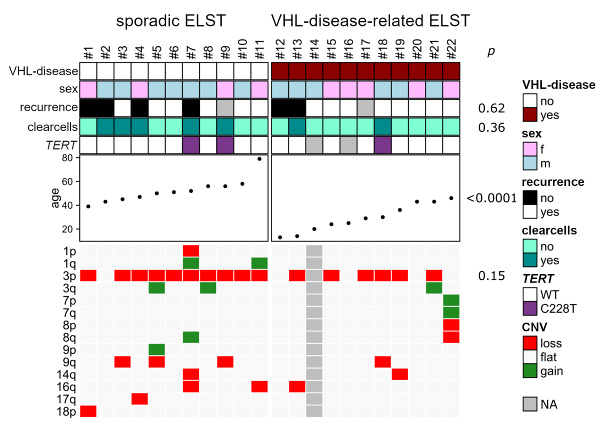
Fig. 2
NOA-03
Free Neuropathol 2:22:12
Establishment and characterization of a meningioma cell model with RagC knockout
R. Diaz Peregrino1, J. Kahr1, N. Waldt1, E. Kirches1, C. Mawrin1
1 Otto-von-Guericke Universität Magdeburg, Institut für Neuropathologie, Magdeburg, Germany
Amino acid sensors are essential for cell growth and carcinogenesis via the mTORC1 pathway. IOMM Lee cells are suitable to study the meningioma pathogenesis, and amino acid sensors like RagC can be investigated in these cells.
The aims of this work are (I) generate a meningioma cell line with RagC deficiency; (II) determine its morphology; (III) characterize it in vitro using cell viability assay, Trypan blue cell counting assay, colony formation assay, and BrdU assay; (IV) and measure its motion.
RagC knockout was achieved through CRISPR-Cas9 system and corroborated by western blot. The area and circularity of IOMM RagCKO and IOMM Scr (scramble control) cells were determined by phalloidin staining. In vitro assays at 24 and 48 hours were performed to Scr and RagCKO cells cultured in normal and leucine-depleted medium. Wound healing assay, velocity, displacement, acceleration, and percentage of moving cells were measured in both cell lines seeded in the standard and leucine-free medium.
Cell area was similar between cell genotypes, but RagCKO cells were more elongated than Scr cells. Based on cell genotype comparison, RagCKO cells were more metabolically active and displayed more cell proliferation than Scr cells. Centered on media comparison, cell numbers and cell proliferation decayed in both cell lines when cultivating them in the leucine-free medium. Only RagCKO cells had a reduction in ATP production under leucine withdrawal. Finally, RagCKO cells exhibited higher motility than Scr cells judged by the motion parameters. Moreover, the motility of RagCKO cells was resistant towards leucine depletion and even increased in comparison to Scr cells.
RagCKO cells displayed increased proliferation and ATP production in leucine-supplied scenarios and accelerated migration under leucine-deprived conditions. Changes in AKT and pp70S6 signaling may be involved in both phenomena. Furthermore, the morphological modifications of RagCKO cells might enhance their motion.
NOA-04
Free Neuropathol 2:22:13
The role of 2-oxoglutarate homeostasis in tumour invasion and metastasis in glioblastoma
A. Alserw1, N. Bögürcü-Seidel1, S. Seidel2, S. Gräf1, S. Zukunft3, I. Fleming3, S. Günther4, M. Looso4, A. Németh1, B. Garvalov1,5, T. Acker1
1 Justus-Liebig-University Gießen, Institute for Neuropathology, Gießen, Germany
2 Institute of Cell Biology and Neuroscience and Buchmann Institute for Molecular Life Sciences (BMLS), Goethe University, Frankfurt a. M., Germany
3 Goethe University Frankfurt, Institute for Vascular Signalling, Frankfurt a. M., Germany
4 Max Planck Institute for Heart and Lung Research, Bioinformatics Core Unit (M.L., P.G.), Bad Nauheim, Germany
5 European Center for Angioscience (ECAS), Department of Microvascular Biology and Pathobiology, Mannheim, Germany
Introduction: Metabolic reprogramming has been associated with tumour invasion and metastasis. Epithelial/proneural-to-mesenchymal transition (EMT/PrMT) is a key process behind cancer cell dissemination, which is regulated by several transcription factors, including Snail. 2-oxoglutarate (2-OG) is a metabolite whose intracellular level depends on the activity of isocitrate dehydrogenase 1 (IDH1). The metabolite acts as a co-substrate for 2-OG-dependent dioxygenases (2-OGDDs) which regulate different cellular activities. While mutant-IDH-induced, 2-OGDD-dependent tumorigenic mechanisms have been described in detail, it is less well understood how wildtype IDH and 2-OG levels control EMT and invasion.
Objectives: We aim to define the homeostatic function of IDH1 and 2-OG in the regulation of EMT and tumour progression primarily in glioblastoma, as well as in breast and lung cancer models.
Materials and Methods: Boyden chamber, western blot, IF, RT-qPCR, RNAseq and metabolite MS assays are employed to determine the role of IDH1 and 2-OG levels on EMT regulators and cell invasion. IDH1 knockdown and several functional assays are used to further explore the underlying mechanisms.
Results: We observed that TGFβ; an established EMT inducer results in IDH1 downregulation, with a concomitant increase in Snail expression and invasion. Strikingly, 2-OG supplementation reverts TGFβ induced Snail at the mRNA and protein level as well as cellular invasion in vitro. In line with a functional role of IDH1 in EMT, IDH1deficiency promotes invasion and metastasis and enhances a cancer stem cell phenotype. Importantly, we identify HIF and mTOR signalling as two crucial mechanistic pathways involved in Snail regulation by 2-OG.
Conclusion: Collectively, our findings emphasize the interplay between metabolism and metastasis and reveal novel mechanistic insights into the role of wildtype IDH and intracellular 2-OG levels in the metabolic control of tumor invasion and EMT/PrMT.
NOA-05
Free Neuropathol 2:22:14
Infratentorial IDH mutant astrocytoma is a distinct subtype
R. Banan1, D. Stichel1, A. Bleck2, B. Hong3, U. Lehmann4, A. Suwala1, A. Reinhardt1, D. Schrimpf1, R. Buslei5, C. Stadelmann-Nessler6, K. Ehlert7, M. Prinz8, T. Acker9, J. Schittenhelm10, D. Kaul11, L. Schweizer12, D. Capper12, P. N. Harter13, N. Etminan14, D. T. W. Jones15, S. M. Pfister16, C. Herold Mende17, W. Wick18, F. Sahm1, A. von Deimling1, C. Hartmann2, D. E. Reuss1
1 Heidelberg University Hospital, Department of Neuropathology, Institute of Pathology, Heidelberg, Germany
2 Hannover Medical School (MHH), Department of Neuropathology, Institute of Pathology, Hannover, Germany
3 Hannover Medical School (MHH), Department of Neurosurgery, Hannover, Germany
4 Hannover Medical School (MHH), Institute of Pathology, Hannover, Germany
5 Klinikum Bamberg, Institute of Pathology, Bamberg, Germany
6 University Medical Center Göttingen, Institute of Neuropathology, Göttingen, Germany
7 University of Greifswald, Department of Pediatric Oncology and Hematology, Greifswald, Germany
8 University of Freiburg, Institute of Neuropathology, Freiburg i. Br., Germany
9 Institute of Neuropathology, University of Gießen, Gießen, Germany
10 University Hospital Tübingen, Department of Neuropathology, Tübingen, Germany
11 Charité – Universitätsmedizin Berlin, Department of Radiation Oncology and Radiotherapy, Berlin, Germany
12 Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
13 Goethe University Frankfurt, Institute of Neurology (Edinger Institute), Frankfurt a. M., Germany
14 University Hospital Mannheim, University of Heidelberg, Department of Neurosurgery, Mannheim, Germany
15 German Cancer Research Center (DKFZ), Pediatric Glioma Research Group, Heidelberg, Germany
16 University Hospital Heidelberg, Department of Pediatric Oncology and Hematology, Heidelberg, Germany
17 University Hospital Heidelberg, Department of Neurosurgery, Division of Experimental Neurosurgery, Heidelberg, Germany
18 University of Heidelberg Medical Center, Neurology Clinic, Heidelberg, Germany
Introduction: Diffuse IDH-mutant astrocytic tumors are rarely diagnosed in infratentorial structures. Only few studies have reported single cases of infratentorial diffuse gliomas harboring IDH mutations, while suffering small case numbers.
Objectives: In this multi-institutional study, we aimed to analyze infratentorial IDH-mutant astrocytomas in a larger series with respect to clinical and molecular parameters.
Patients and methods: Tumor samples of patients with diffuse infratentorial astrocytomas from different German neuropathology departments were investigated using immunohistochemistry, pyrosequencing, genome-wide DNA methylation analysis and NGS panel sequencing. Overall survival data were assessed using Kaplan–Meier method.
Results: We identified a series of 43 infratentorial IDH-mutant astrocytomas from 42 patients. About 80% of IDH mutations were of non-IDH1-R132H variants which are rare in supratentorial astrocytomas. Most frequently, IDH1-R132C/G and IDH2-R172S/G mutations were present. Moreover, ATRX-loss and MGMT promoter methylation typically associated with IDH mutations in supratentorial astrocytomas, were significantly less frequent in the infratentorial compartment. Panel sequencing revealed two samples with IDH1-R132C/H3F3A-K27M co-mutations. Methylation analysis and copy number profiling provided further evidence for a molecular distinctiveness of these tumors. Clinical outcome of patients with infratentorial IDH-mutant astrocytomas was significantly better than in patients with diffuse midline gliomas, H3K27M-mutant (p < 0.005) and poorer than in those with supratentorial IDH-mutant astrocytomas (p = 0.028).
Conclusion: The presented data highlight the very existence and distinctiveness of infratentorial IDH-mutant astrocytomas implying that molecular testing is critical for detection of these tumors, since many of these tumors cannot be identified by immunohistochemistry applied for the mutated IDH1-R132H protein or loss of ATRX.
NOA-06
Free Neuropathol 2:22:16
DNA methylation profiling and molecular grading of stereotaxic brain tumor biopsies
K. Filipski1,2,3, J. Hench4, M. Armbrust1, P. S. Zeiner2,3,5, T. I. Hartung1, E. Steidl6, J. Quick-Weller7, T. Fenton8, J. P. Steinbach2,3,5, M. Czabanka7, D. Capper9,10, S. Frank4, K. H. Plate1,2,3, M. T. Forster7, P. N. Harter1,2,3
1 University Hospital Frankfurt, Neurological Institute (Edinger Institute), Frankfurt a. M., Germany
2 German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany
3 Frankfurt Cancer Institute (FCI), Frankfurt a. M., Germany
4 Basel University Hospital, Department of Neuropathology, Institute of Pathology, Basel, Switzerland
5 University Hospital Frankfurt, Dr. Senckenberg Institute of Neurooncology, Frankfurt a. M., Germany
6 University Hospital Frankfurt, Institute of Neuroradiology, Frankfurt a. M., Germany
7 University Hospital Frankfurt, Department of Neurosurgery, Frankfurt a. M., Germany
8 University of Kent, School of Bioscience, Kent, United Kingdom
9 Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
10 German Cancer Consortium (DKTK), Partner Site Berlin, German Cancer Research Center (DKFZ), Heidelberg, Germany
Introduction: DNA methylation profiling of CNS tumors emerges as a powerful tool to support and refine histological diagnoses. Tumor entity-specific DNA methylation signatures allow for alignment with methylation classes and copy number profiling (CNP), the latter being of particular interest in terms of molecular grading regarding the upcoming WHO classification. When gross tumor resection is precluded, stereotaxic biopsies might not be representative of the bulk tumor, underlining the need for objective molecular diagnostic parameters.
Objective: Here we investigated the diagnostic potential and accuracy of DNA methylation profiling and molecular grading on small stereotaxic brain tumor biopsies.
Materials & Methods: FFPE samples of 237 patients were subjected to DNA methylation analysis including the Heidelberg Brain Tumor Classifier, CNP and tumor deconvolution using MethylCIBERSORT.
Results: Median DNA concentration was 6.26 ng/µl. 90.5% of STX samples (n=232) allocated to previously defined DNA methylation classes, with 61.6% reaching classifier scores of > 0.84. Combined gain of chromosome 7 and loss of chromosome 10 or EGFR amplification were detectable in 61% and 38% of IDH wildtype glioma (n=156), respectively. 64.5% of histological lower grade, IDH wildtype gliomas (n=31) were eligible for upgrading to molecular glioblastoma. 58% of IDH mutant gliomas (n=31) presented with 1p/19q codeletion and 23% with homozygous deletion of CDKN2A/B. Recursive partitioning pointed at a minimum of 29.42% cancer cells for a 0.83 probability to reach a matching calibrated score in glioblastoma, IDH wildtype. A DNA input threshold of 3.53 ng/µl yielded matching DNA methylation classes with a probability of 0.69.
Conclusion: Our findings demonstrate broad applicability of DNA methylation profiling and molecular grading to challengingly small, ill-defined brain tumor specimens with a special impact on grading of histologically lower grade, non-IDH mutant diffuse gliomas.
NOA-07
Free Neuropathol 2:22:18
Prediction of meningioma methylation classes using AI-assisted digital histopathology
J. Sehring1, H. Dohmen1, M. Manke1, D. Amsel1, A. Németh1, A. Mukhopadhyay2, T. Acker1
1 Justus-Liebig-University Gießen, Institute of Neuropathology, Gießen, Germany
2 Technical University Darmstadt, Medical and Environmental Computing, Darmstadt, Germany
Introduction: Current studies suggest that DNA methylation-based molecular classification of meningioma has a higher predicting power for tumor recurrence than the current histology-based WHO classification. However DNA methylation profiling is costly, time-consuming and not widely available. Therefore, an AI-histology-based prediction of the six DNA methylation classes would be very beneficial and could additionally complement the molecular classification. Deep neural networks and their visual decoding power offer a promising tool to address this problem.
Objectives: Our goal is to predict meningioma methylation classes using AI-assisted digital histopathology.
Materials and methods: H&E stained histological slides were digitized with a Hamamatsu Nanozoomer S360 to obtain Whole Slide Images(WSI). Multiple image patches per patient were extracted and encoded into feature space using a pre-trained ResNet50. Those features were aggregated in a multiple instance learning setting using an attention network to predict the methylation class.
Results: We have collected WSI data of 167 patients so far. Most specimens belong to the most frequently diagnosed methylation subgroups, namely benign-1 (n=36), benign-2 (n=55) and intermediate-A (n=60). Three separate two-class classifications (benign-1 vs. benign-2, benign-1 vs. intermediate-A and benign-2 vs. intermediate-A) are currently under development. Patients were allocated into training, validation and test sets (0.7/0.1/0.2) and we evaluated the patient-wise balanced accuracy on the test set in a 5-fold cross-validation. In addition, the attention weights for the image patches of a WSI were visualized for interpretability.
Conclusion: We employ a framework for the prediction of methylation classes based on histological features. Visualization is used for an enhanced interpretability of the black-box network. This AI-framework may support, complement, and accelerate meningioma classification in the future.
Muscle & Nerve Diseases
MND-01
Free Neuropathol 2:22:19
In the spotlight: NK cell receptors in inclusion body myositis
C. Nelke1, M. Pawlitzki2, T. Ruck1
1 University Hospital Duesseldorf, Deparment for Neurology, Neurology, Düsseldorf, Germany
2 University Hospital Münster, Department of Neurology with Intitute of Translational Neurology, Münster, Germany
Introduction: Inclusion body myositis (IBM) is the most common inflammatory idiopathic myopathy (IIM) of old age. As of yet, effective treatment approaches are lacking. A deeper immunological understanding is necessary to overcome this roadblock. As natural killer (NK) cells orchestrate the extent of inflammatory responses by cytolytic properties in IIM capable of inducing bystander self-tissue damage, we aimed to characterize NK cell patterns in IBM.
Objectives: To determine NK cell patterns in IBM and identify drivers of NK cell activation.
Patients & methods: We included 20 IBM patients and 20 age and sex matched non diseased controls (NDC). Peripheral blood was collected and peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation. Differences between groups were analyzed using unpaired Student"s t test or Mann–Whitney-U test as appropriate. Anti-cN-1A-antibodies were detected in patient serum using the EUROIMMUN immunoassay.
Results: The activated CD56dim population was reduced in peripheral blood of IBM patients as compared to controls. Comparing surface expression as measured by mean fluorescence intensity, we observed a significant increase of NKG2A and NKG2D levels on the CD56dim NK cell subset in IBM patients as compared to NDC. Other surface receptors remained unchanged. Next, we were interested whether the antibody status of IBM patients affects expression of surface receptors. Consequently, comparison of anti-cN-1A positive and anti-cN-1A negative patients revealed that NKG2A level do not differ, while increased NKG2D levels were associated with anti-cN-1A-ab positivity.
Conclusion: Here, we report distinct NK cell patterns in IBM patients. Activated CD56dim NK cells are reduced in peripheral blood as compared to NDC. NKG2A and NKG2D levels are increased on CD56dim NK cells. Lastly, increased NKG2D levels were associated with anti-cN-1-ab serostatus.
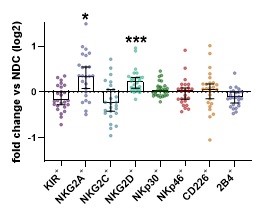
Fig. 1
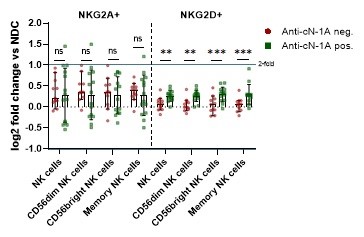
Fig. 2
MND-02
Free Neuropathol 2:22:21
Mutations in BVES can lead to early onset of cardiac symptoms without clinical signs of muscle dystrophy in children and adolescents
A. Gangfuß1, A. Hentschel2, M. Gonzales3, A. Schönecker4, A. Töpf5, A. Sickmann2, A. Nishimura6, U. Schara-Schmidt1, A. Hahn7, A. Roos1, A. Schänzer6
1 University Duisburg-Essen, Department of Pediatric Neurology, Centre for Neuromuscular Disorders, Centre for Translational Neuro- and Behavioral Sciences, Essen, Germany
2 Leibniz-Institut für Analytische Wissenschaften - ISAS - e.V., Dortmund, Germany
3 Justus-Liebig-University Gießen, Pediatric Heart Center, Gießen, Germany
4 University Duisburg-Essen, Department of Pediatric Cardiology, Essen, Germany
5 Newcastle University and Newcastle Hospitals NHS Foundation Trust, The John Walton Muscular Dystrophy Research Centre, Translational and Clinical Research Institute, Newcastle upon Tyne, United Kingdom
6 Newcastle University and Newcastle Hospitals NHS Foundation Trust, The John Walton Muscular Dystrophy Research Centre, Translational and Clinical Research Institute, Newcastle upon Tyne, United Kingdom
7 Justus-Liebig-University Gießen, Department of Child Neurology, Gießen, Germany
Introduction: Limb-girdle muscular dystrophies occur in children and adults and may be associated with a high variability of the clinical phenotype. In some subtypes, cardiac involvement is the first and thus key symptom. Recently, Blood vessel endothelial substance (BVES), a gene encoding Popeye domain containing protein 1 (POPDC1), has been implicated as a causative for limb-girdle dystrophy type R25 (LGMDR25), associated with cardiac arrhythmia and variable skeletal muscle involvement. Until today, only 10 adult patients with this rare autosomal recessive LGMD have been described.
Objectives: With this study, we aim to broaden the phenotypic spectrum and improve the pathophysiological understanding of LGMDR25.
Materials & methods: Hence, we describe the clinical phenotype of four affected children of two families with homozygous variants (c.457>T; p.Gln153Ter and c.578T>G, p.Ile193Ser) in Blood vessel endothelial substance (BVES). Additionally, we performed detailed muscle biopsy analysis from two affected patients including immunofluorescence, electron microscopic and proteomic studies.
Results: Highly elevated creatine kinase and cardiac involvement are present in all patients. Muscle weakness was not present in any of the patients, considering the oldest being 19 years old. However, histological and ultrastructural analysis revealed a myopathy. Immunohistochemistry showed an absence of sarcolemnal expression of BVES confirming the diagnosis. Additional proteomic analyses were indicative for an impairment of the calcium balance and thus a potential influence on the contractile apparatus.
Conclusion: To conclude, LGMDR25 should be considered in children with cardiac symptoms and highly elevated creatine kinase, even in the absence of muscle weakness. A muscle biopsy is helpful to confirm the diagnosis and to increase the understanding of the disease pathology.
Neurodegeneration / COVID-19 and Neuropathology
ND-01
Free Neuropathol 2:22:23
Combining postmortem single cell analysis with an induced pluripotent stem cell model to study dysregulated pathways in frontotemporal dementia
O. Al Dalahmah1, R. Kühn1, T. Nguyen1, M. DeTure2, M. Siegelin1, D. Dickson2, J. P. Vonsattel1, J. Goldman1, P. Canoll1, G. Hargus1
1 Columbia University, Department of Pathology and Cell Biology, New York, NY, United States
2 Mayo Clinic, Jacksonville, FL, United States
Introduction: Frontotemporal dementia (FTD) is a group of early-onset dementias leading to an impairment of behavior, language and cognition. FTD can be caused by mutations in the MAPT gene encoding the microtubule-associated protein tau resulting in pronounced atrophy of the frontal and temporal lobes, basal ganglia and brain stem areas.
Objectives: Currently, the underlying mechanisms of neurodegeneration are not clearly understood and curative options do not exit.
Materials & Methods: Here, we performed single nucleus RNA sequencing (snRNA-seq) on postmortem brain tissue from FTD patients carrying the MAPTN279K mutation and from healthy control individuals to identify dysregulated pathways in patient cells at single cell resolution.
Results: We found significant changes in pathways related to cell metabolism and neuroinflammation in patient neurons that we tested further in a stem cell model of FTD using patient- and control individual-derived induced pluripotent stem cells (iPSCs). Patient iPSC-derived neurons with the MAPTN279K mutation demonstrated tau pathology, an impairment of neurite outgrowth and an increased but reversible oxidative stress response. FTD neurons also showed altered metabolic profiles with an increased basal mitochondrial respiration and increased ATP production indicating an increased energy demand in these cells. Interestingly, FTD neurons also had a significant effect on the survival of host neurons and on glial cell responses when transplanted into the brains of immunocompromised mice indicating a potential immunomodulatory role of patient neurons in vivo.
Conclusion: These findings demonstrate that a combinatorial approach applying snRNA-seq on patient brain tissue and a dynamic iPSC model comprises a powerful tool to identify disease phenotypes in neural cells at risk in FTD. Such stem cell model could also be used as a cellular platform for high-throughput drug screening assays to identify potential therapeutic targets in FTD.
ND-02
Free Neuropathol 2:22:24
Deep learning assisted quantitative assessment of histopathological markers of Alzheimer"s disease and cerebral amyloid angiopathy
V. Perosa1,2, A. Scherleck3, M. Kozberg1, L. Smith4, T. Westerling-Bui4, C. A. Auger5, S. Vasylechko6, S. M. Greenberg1, S. J. van Veluw1,5
1 Massachusetts General Hospital, 1J. Philip Kistler Stroke Research Center, Boston, MA, United States
2 Otto-von-Guericke University, Department of Neurology, Magdeburg, Germany
3 Rush University Medical Center, 3Rush Alzheimer Disease Center, Chicago, IL, United States
4 Aiforia Inc, Cambridge, MA, United States
5 Massachusetts General Hospital, MassGeneral Institute for Neurodegenerative Disease, Charlestown, MA, United States
6 Boston Children's Hospital, Computational Radiology Laboratory, Boston, MA, United States
Introduction: Traditionally, analysis of neuropathological markers in neurodegenerative diseases has relied on visual assessments of stained sections. Resulting semiquantitative scores often vary between individual raters and research centers, limiting statistical approaches. To overcome these issues, we have developed six deep learning-based models, that identify some of the most characteristic markers of Alzheimer"s disease (AD) and cerebral amyloid angiopathy (CAA).
Methods: Deep learning-based models were trained to differentially detect parenchymal amyloid β (Aβ)-plaques, vascular Aβ-deposition, iron and calcium deposition, reactive astrocytes, microglia, as well as fibrin extravasation. The models were trained on digitized histopathological slides from brains of patients with AD and CAA, using a workflow that allows neuropathology experts to train convolutional neural networks (CNNs) on a cloud-based graphical interface.
Results: Validation of all models indicated a very good to excellent performance compared to three independent expert human raters. Furthermore, the Aβ and iron models were consistent with previously acquired semiquantitative scores in the same dataset and allowed the use of more complex statistical approaches.
Discussion: The presented workflow is easy for researchers with pathological expertise to implement and is customizable for additional histopathological markers. The implementation of deep learning-assisted analyses of histopathological slides is likely to promote standardization of the assessment of neuropathological markers across research centers, which will allow specific pathophysiological questions in neurodegenerative disease to be addressed in a harmonized way and on a larger scale.
ND-03
Free Neuropathol 2:22:25
Exercise ameliorates Alzheimers pathology across generations
A. Herring1, Y. Münster1, N. Kurapati1, R. Khadzhiev1, K. Keyvani1
1 University Duisburg-Essen, Institute of Neuropathology, Essen, Germany
Introduction: An active lifestyle reduces the risk of dementia and cognitive decline in Alzheimer"s diseases (AD) patients. Animal studies have shown that environmental enrichment increases the cognitive reserve and interferes with the pathomechanisms of AD over a very extended period of ontogenesis, from in utero stimulation via maternal running during pregnancy until late exercise in a full-blown stage disease.
Objectives: Here we asked whether protective effects of exercise against AD can be inherited across several generations and if so, what epigenetic modifications enable transgenerational transmission.
Materials & methods: In the founder generation (F0), TgCRND8 mice (disease onset around postnatal day 90, P90) and wildtype littermates had either access to voluntary wheel running for six months, starting at P30, or were housed without exercise (control). From P90, either exercised mice were mated or mice that experienced a sedentary life style. The offspring was solely housed under control conditions and crossed until the third progenitor generation. The progeny (F1-F3) was phenotyped for memory and explorative behaviour as well as amyloid beta (Aβ) pathology in an advanced stage disease (P210). To detect epigenetic transmitters, we determined the cerebral expression profile of microRNAs involved in Aβ metabolism.
Results: We here show that great-grandparental running reduced Aβ plaque burden, enhanced cerebral levels of the Aβ efflux transporter MDR1 and further increased recognition memory and explorative behaviour in transgenic mice until the F3 generation. Further, the cerebral expression of microRNAs that promote Aβ pathology (miRNAs 342-3p, 16-5p, 9-5p) was strongly reduced following ancestral exercise.
Conclusion: We conclude that protective effects of exercise can propagate across generations. Ongoing experiments aim to decode further epigenetic modifications that enable transgenerational inheritance.
Neurooncology Pediatrics
NOP-01
Free Neuropathol 2:22:26
Co-activation of Sonic hedgehog and Wnt signaling in murine retinal precursor cells drives ocular lesions resembling intraocular medulloepithelioma
M. Dottermusch1,2, P. Sumislawski1, J. Krevet3,4, H. Voß5, M. Middelkamp1, H. Bartsch6, K. Sotlar6,7, A. Korshunov8, M. Glatzel1, U. Schüller1,4,9, J. Neumann1,2
1 University Medical Center Hamburg-Eppendorf, Institute of Neuropathology, Hamburg, Germany
2 University Medical Center Hamburg-Eppendorf, Center for Molecular Neurobiology (ZMNH), Hamburg, Germany
3 Ludwig-Maximilians-University Munich, Center for Neuropathology, München, Germany
4 Research Institute Children’s Cancer Center Hamburg, Hamburg, Germany
5 University Medical Center Hamburg-Eppendorf, Institute of Clinical Chemistry and Laboratory Medicine, Hamburg, Germany
6 Ludwig-Maximilians-University Munich, Department of Pathology, München, Germany
7 Paracelsus Medical University, Department of Pathology, Salzburg, Austria
8 German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Clinical Cooperation Unit Neuropathology (G380), Heidelberg, Germany
9 University Medical Center Hamburg-Eppendorf, Department of Pediatric Hematology and Oncology, Hamburg, Germany
Introduction: Intraocular medulloepithelioma (IO-MEPL) is a rare embryonal ocular neoplasm, which prevalently occurs in young children and is commonly treated by enucleation of the eye. IO-MEPLs share common histomorphological features with variants of CNS embryonal tumors with multilayered rosettes (ETMR), referred to as intracranial medulloepitheliomas. While sonic hedgehog (Shh) and Wnt signaling pathways are crucial for ETMR pathogenesis, the impact of these pathways on human IO-MEPL development is unclear.
Objectives: Our objective in this study was to explore the relevance of SHH and WNT signaling in human IO-MEPLs and demonstrate the effect of simultaneous Shh and Wnt activation in mouse retinal precursor cells.
Materials & methods: Using Nanostring technology, gene expression data was obtained from FFPE tumor samples of 8 human IO-MEPLs, as well as 16 intracranial embryonal tumors comprising ETMRs, SHH-medulloblastomas (MBs), WNT-MBs and Group 4-MBs. In order to unravel the function of Shh and Wnt signaling for IO-MEPL pathogenesis in vivo in a time point and cell specific manner, the tamoxifen inducible creERT2-lox system was utilized to activate Shh and Wnt signaling in Sox2- or Rax- expressing retinal precursor cells.
Results: IO-MEPLs and ETMRs displayed similar gene expression patterns and significant simultaneous overrepresentation of both SHH and WNT target genes. Co-activation of both pathways in early Sox2- or Rax- expressing precursor cells resulted in infiltrative ocular lesions that displayed extraocular expansion. Histomorphological, immunohistochemical and molecular features of these lesions revealed strong concordance with those of human IO-MEPLs.
Conclusion: We report a relevant role of WNT and SHH signaling in IO-MEPL and report a mouse model for this rare disease, setting the foundation for a targeted therapeutic approach with the aim of more commonly feasible eye salvage in affected patients.
NOP-02
Free Neuropathol 2:22:28
Characterisation of spinal diffuse midline gliomas
L. Stegat1, U. Schüller1,2,3, A. Wefers1
1 University Medical Center Hamburg-Eppendorf, Institute of Neuropathology, Hamburg, Germany
2 University Medical Center Hamburg-Eppendorf, Department of Pediatric Hematology and Oncology, Hamburg, Germany
3 Research Institute Children’s Cancer Center Hamburg, Hamburg, Germany
Introduction: Diffuse midline gliomas (DMGs) are malignant gliomas that grow in midline structures of the central nervous system. Due to their aggressive and diffuse growth and a two-year survival rate of less than 10%, DMGs are graded as WHO grade IV. Depending on the localization, median age of patients is about 11-20 years. Genetically, tumors are defined by a K27M-mutation in one of the highly homologous genes encoding histone protein H3. While some studies state that the global DNA methylation pattern is correlated with the mutated histone gene, others suggest that it differs between DMGs of different localisations.
Objectives: Since DMGs most frequently occur in pons and thalamus, comparatively little is known about spinal DMGs. Therefore, we histologically, molecularly and clinically characterised spinal DMGs and analysed in which aspects they differ from DMGs of other localizations as this may have diagnostical and/or clinical implications.
Materials & methods: Our cohort currently consists of 17 spinal DMGs plus DMGs of different localisations. Histological and immunohistochemical analysis as well as molecular analyses (DNA methylation, DNA panel sequencing) were done from FFPE tissue. Clinical data were analysed with Prism.
Results: Spinal DMGs were histologically very heterogeneous, both with regard to different areas of single tumors as well as when comparing different tumors. Preliminary data suggest that there may be some differences to DMGs of different localisations, e.g. with regard to cell differentiation. First t-SNE analyses of DNA methylation data did not indicate a separation of spinal DMGs from those of different localisations. 10/11 spinal DMGs sequenced so far were H3F3A K27M-mutant, while one tumor had a HIST1H3B K27M mutation. Mean age was 22 years.
Conclusion: First analyses suggest slight histological differences of spinal DMGs compared to those of other localisations. Further histological, molecular and clinical analyses are ongoing.
Postersession I – Neuroinflammation
PI-01
Free Neuropathol 2:22:29
Neurofilament light chains in serum are a biomarker for the acute axonal damage in MS lesions: A histological-serological correlative study
A. S. Beutler1, N. Kruse1, L. Stork1, M. Gloth1, C. Stadelmann-Nessler1, W. Brück1, I. Metz1
1 University Medical Center Göttingen, Georg-August-University, Institute of Neuropathology, Göttingen, Germany
Introduction: Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS, associated with a variable axonal damage. The axonal damage is responsible for the irreversible clinical disability, and within the CNS no relevant neuroaxonal regeneration occurs. Thus, in MS patients it is of utmost importance to monitor and hinder the axonal damage from the earliest stages on. A promising biomarker are neurofilament light chains measured in serum (sNfL). However, sNfL do not provide any information regarding the localization of the neuroaxonal damage, and multiple factors influence sNfL levels.
Objectives: To perform a histopathological-serological correlative study to show the pathological correlate of elevated sNfL levels in MS patients.
Patients and Methods: 106 MS subjects with biopsy tissue and blood sample available were characterized for their acute axonal damage with the anti-APP staining and the chronic axonal damage with the Bielschowsky silver staining. Results were correlated with the neurofilament light chain levels in serum measured with the single molecular array (SiMoA) technique.
Results: High sNfL levels were present in biopsied MS patients (median 59 pg/ml) compared to healthy controls (19 pg/ml), and levels were influenced by the age of patients, relapses prior blood sampling as well as the time interval after biopsy. Correlating histological parameters with sNfl, multiple regression analyses showed that the acute axonal damage within early active MS lesions significantly contributed to the sNfL (p<0.01), but not the axonal density. Importantly, sNfL correlated with the clinical disability at biopsy as well as at last follow-up.
Conclusion: sNfL mirror the acute axonal damage in early MS lesions as shown by histology, and correlate with the clinical disability of patients. They are thus a promising biomarker to monitor the axonal damage ex vivo in MS patients.
This study was sponsored by Novartis Pharma GmbH.
PI-02
Free Neuropathol 2:22:30
MGMT-promoter methylation in non-neoplastic diseases of the central nervous system
S. Teuber-Hanselmann1, K. Worm2, A. Junker1
1 Universitätsklinikum Essen, Institut für Neuropathologie, Essen, Germany
2 Universitätsklinikum Essen, Institut für Pathologie, Essen, Germany
Introduction: O6-methylguanine-DNA-methyltransferase (MGMT) plays a pivotal role in cellular defense by repairing alkylated DNA. Hypermethylation of the MGMT promoter region results in gene silencing, an effect seen in various tumors, like gliomas. MGMT hypermethylation was postulated to be attributed to cancers or to malignant transformation of precancerous lesions and that consequently it could be used as a tumor biomarker. But while MGMT hypermethylation was also detected in various peripheral inflammatory diseases, it has not yet been investigated in non-neoplastic CNS diseases.
Objectives: To investigate MGMT promoter methylation levels in various non-neoplastic CNS diseases.
Methods: MGMT promoter methylation and expression levels of DNA demethylases TET1 and TET2 were investigated via pyrosequencing and immunohistochemistry on human autopsy or biopsy FFPE tissue samples with PML (10), MS (28), toxoplasmosis (6), CMV (1), HSV1 (1) or HIV (2) infection, mycotic encephalitis (4), brain abscesses (3), central pontine / extrapontine myelinolysis (CPM/EPM) (8), Wallerian degeneration (3) and controls without CNS pathologies (8).
Results: Slight MGMT hypermethylation was seen in non-neoplastic CNS diseases, all of which were associated with damaged myelin sheaths, i.e. inflammatory and demyelinating (MS and PML), as well as non-inflammatory metabolic or degenerative CNS diseases (CPM/EPM, Wallerian degeneration), while there were no associations with infectious non-demyelinating diseases or distinct pathogens. Reduced expression levels of TET1 could possibly be the cause for MGMT hypermethylation.
Conclusion: MGMT hypermethylation is not restricted to neoplastic or infectious diseases but occurs in chronic CNS diseases that lead to damage of the myelin sheath in various ways. While this gives new insights into epigenetic and pathophysiological processes involved in de-/remyelination, it also reduces the specificity of MGMT hypermethylation as a tumor biomarker.
PI-03
Free Neuropathol 2:22:31
Chronic retrotransposon expression in mouse glial cells is associated with spatial learning deficits without an overt inflammatory response
R. Sankowski1, G. Monaco1, M. Prinz1
1 Universitätsklinikum Freiburg, Institut für Neuropathologie, Freiburg i. Br., Germany
Introduction: Retrotransposons are mobile genomic elements comprising approximately 40 percent of the human and mouse genomes. Activation of retrotransposons has been associated with an inflammatory response in the brain and peripheral tissues. We have previously shown that chronic retrotransposon activation in B cell deficient mice was associated with astrogliosis and spatial learning deficits.
Objective: We were wondering about cell-type dependent retrotransposon expression in the B cell deficient mouse model of constitutive retrotransposon activation.
Materials & Methods: Here, we have utilised single-nucleus and single-cell RNA-Sequencing to profile the expression of retrotransposons in hippocampi and cortices of B cell deficient mice.
Results: We could identify increased expression of long-terminal repeat and non-long terminal repeat retrotransposons in microglia and astrocytes compared to other cell types. Surprisingly, these expression changes were not associated with an increased expression of genes encoding inflammatory mediators. We confirmed these results using bulk RNA sequencing.
Conclusion: Our findings underscore the ability of brain tissues to attenuate an inflammatory response despite continuing presence of an immunogenic agent with direct therapeutic implications for unresolving chronic inflammatory conditions.
Postersession II – Neurooncology
PII-01
Free Neuropathol 2:22:32
DNA methylation-based classification of sinonasal tumors
P. Jurmeister1, S. Glöß2, R. Roller3, S. Schmid2, R. Fritz3, A. Thieme2, C. Friedrich3, I. Hoffmann3, M. Leitheiser3, P. Mertins4, F. Klauschen1, D. Capper2
1 Ludwig-Maximilians-University Munich, Institute of Pathology, München, Germany
2 Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
3 Charité – Universitätsmedizin Berlin, Institute of Pathology, Berlin, Germany
4 Max-Delbrück-Centrum für Molekulare Medizin, Berlin, Germany
Introduction: The histopathological diagnosis of sinonasal tumors is challenging as it encompasses a heterogenous spectrum of diverse differential diagnoses as well as poorly defined, disputed entities such as sinonasal undifferentiated carcinomas (SNUCs).
Objectives: In this study, we aimed to develop a DNA methylation-based classification algorithm for sinonasal tumors and to elucidate the molecular background of SNUCs.
Materials & Methods: We established a cohort of DNA methylation profiles from 374 sinonasal tumor specimens, covering 17 tumor entities and normal control tissue. Furthermore, we performed DNA sequencing and mass spectrometry-based proteomics of selected cases.
Results: A machine learning algorithm was able to reliably classifiy sinonasal tumors based on their DNA methylation profiles with clinical-grade accuracy. We further showed that tumors with SNUC morphology can be assigned to four molecular classes defined by distinct epigenetic, mutational and proteomic profiles. This included two classes with clear neuroendocrine differentiation in proteomics analysis which were characterized by either IDH2 mutations or SWI/SNF chromatin remodeling complex (SMARCA4/ARID1A) mutations and overall favorable clinical course. The third group comprised highly aggressive tumors, driven by SMARCB1-deficiency. The fourth SNUC class contained tumors that actually represented previously misclassified adenoid-cystic carcinomas, which was supported by recurrent MYB translocations and the resemeblance of these specimens with serous cell of submucosal glands in proteomics analysis.
Conclusions: Our DNA methylation-based machine learning algorithm could greatly improve the diagnostics of challenging sinonasal tumors. Furthermore, we provide solid proof that sinonasal tumors are not as undifferentiated as their current terminology implies, but rather represent distinct molecular groups with at least two classes showing clear neuroendocrine differentiation.
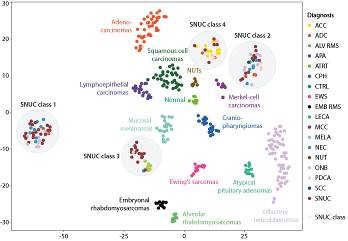
Fig. 1
PII-02
Free Neuropathol 2:22:34
Deep Learning aided mitotic figure count in human meningiomas based on the open-source software SlideRunner
S. Jabari1, C. Bertram2, R. Coras1, A. Maier3, I. Blümcke1, M. Aubreville4
1 University Hospitals Erlangen, Institute for Neuropathology, Erlangen, Germany
2 Veterinärmedizinische Universität Wien, Institut für Pathologie, Vienna, Austria
3 Lehrstuhl für Mustererkennung, FAU, Erlangen, Germany
4 Image Understanding and Medical Application of Artificial Intelligence, Ingolstadt, Germany
Introduction: The World Health Organization (WHO) classification of brain tumors describes three grades of
Meningioma: WHO I-III. A major factor that distinguishes the classes is the number of mitotic figures. Detecting and counting mitotic figures reliably in meningioma is, therefore, an essential part of the diagnostic workup.
Objectives: However, detecting mitotic figures quickly, reliably, and comprehensively in a tissue section is a major challenge. For this reason, we developed a technical assistance system that detects mitotic figures on whole-slide-images and visualizes these as a heat map showing the mitotic density in almost real-time.
Patients & methods: Meningioma samples were retrospectively collected from the Dept. of Neuropathology Erlangen, Germany. All samples were rereviewed by an expert neuropathologist and subsequently digitized using the Hamamatsu S60 digital slide scanner. The slides have been completely annotated for mitotic figures and we provide secondary annotations for mitotic figure look-alikes. Additionally to a blinded two-expert manual annotation, we developed an algorithm-aided dataset, where potentially missed mitotic figures were detected by a deep neural network and subsequently assessed by a human expert.
We used a pretrained deep-neuronal network approach (RetinaNet) to validate our data set.
Finally, a plugin for SlideRunner, an open-source software, was built for convenience enabling real-time identification of mitotic figures in virtual slides.
Results: Out of 69 whole slide images of the complete data set, 46 were randomly assigned to the training set and 23 to be the test set. A preliminary evaluation yielded an F1 score of 0.659.
Conclusion: Error-prone and tedious mitotic figure counts and region of interest identification may be assisted in the future with the help of modern deep learning algorithms integrated into a user-friendly software suite.
PII-03
Free Neuropathol 2:22:35
Intracranial Ependymoma in Adults, a Methylome-based Analysis
M. Träger1, L. Schweizer2,3, P. Vajkoczy4, K. Fukuoka5, K. Ichimura5, U. Schüller6, L. Dührsen7, M. Müther8, W. Paulus9, C. Thomas9, J. Schittenhelm10, F. Eckert11, K. M. Niyazi12, D. F. Fleischmann12, M. Dorostkar13, P. Feyer14, S. A. May15, D. Moskopp16, H. Badakhshi17, S. Wecker17, C. Radke18, J. Walter19, D. Capper2,3, D. Kaul1,3
1 Charité – Universitätsmedizin Berlin, Department of Radiation Oncology, Berlin, Germany
2 Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
3 German Cancer Consortium (DKTK), Partner Site Berlin, German Cancer Research Center (DKFZ), Heidelberg, Germany
4 Charité – Universitätsmedizin Berlin, Department of Neurosurgery, Berlin, Germany
5 National Cancer Center Research Institute, Division of Brain Tumor Translational Research, Tokyo, Japan
6 University Medical Center Hamburg-Eppendorf, Institute of Neuropathology, Hamburg, Germany
7 University Medical Center Hamburg-Eppendorf, Department of Neurosurgery, Hamburg, Germany
8 University Hospital Münster, Department of Neurosurgery, Münster, Germany
9 University Hospital Münster, Institute of Neuropathology, Münster, Germany
10 University Hospital Tübingen, Department of Neuropathology, Tübingen, Germany
11 University Hospital Tübingen, Department of Radiation Oncology, Tübingen, Germany
12 University Hospital, LMU Munich, Department of Radiation Oncology, München, Germany
13 Ludwig-Maximilians-University Munich, Center for Neuropathology, München, Germany
14 Vivantes Hospital Neukölln, Berlin, Germany
15 Klinikum Chemnitz, Department of Neurosurgery, Chemnitz, Germany
16 Vivantes Klinikum Im Friedrichshain, Department of Neurosurgery, Berlin, Germany
17 Ernst Von Bergmann Medical Center, Academic Teaching Hospital of Humboldt University Berlin (Charité), Department of Clinical Radiation Oncology, Potsdam, Germany
18 Ernst Von Bergmann Medical Center, Academic Teaching Hospital of Humboldt University Berlin (Charité), Department of Pathology, Potsdam, Germany
19 Medical Center Saarbruecken, Department of Neurosurgery, Saarbrücken, Germany
Introduction: Recently DNA methylation profiling became widely accepted as a method to identify biologically distinct subgroups of ependymoma. However, due to the rarity of these tumors in adults, previous studies have failed to achieve a sufficient number of patients to characterize the landscape of DNA methylation profiles, their clinical characteristics and prognosis in this age group.
Objectives: We investigated DNA methylation profiles of adult intracranial ependymoma collected from 12 different centers and sought to correlate molecular subgroups to clinical characteristics and outcome.
Materials and methods: Tumors from 157 adult patients histologically classified as WHO grade II or III intracranial ependymoma between 1990 and 2020 were subjected to DNA methylation profiling using the Illumina Human Methylation 450k or EPIC Bead Chip platform. Profiles were matched with the DKFZ Brain Tumor Classifier and detected molecular subgroups were correlated with overall survival (OS) and progression free-survival (PFS).
Results: In 68.8% (108/157) of cases, the suggested methylation class confirmed diagnosis of an ependymal tumor with the additional assignment of a molecular subgroup (49 PFB, 36 SE, 17 RELA, 3 PFA, 2 spinal EPN and1 myxopapillary EPN) whereas 5.7% (9/157) were not compatible with the initial diagnosis of ependymoma. The remaining 25.5% (40/157) did not reach a calibrated score ≥0.9 and were considered not classifiable.
Median follow-up was 54 months. Five and 10-year PFS rates were 54.1% and 40.6% for SE, 65.2% and 37.1% for PF-B, 49% and 49% for RELA tumors.
The 5- and 10-year OS rates were 70.1% and 49.1%for SE, 95.4% and 85.5% for PF-B and 100% and 75% for RELA tumors.
Conclusion: This cohort of adult intracranial ependymoma comprises various molecular subgroups and even different tumor entities. Most patients with ependymal tumors confirmed by DNA methylation profiling show a favorable prognosis. However, factors that affect survival need to be determined.
Poster session III – Muscle & Nerve
PIII-01
Free Neuropathol 2:22:37
Imaging of extended tissue areas by automated large-scale electron microscopy
C. Dittmayer1, H. H. Goebel1,2, F. L. Heppner1, W. Stenzel1
1 Charité – Universitätsmedizin Berlin, Department of Neuropathology, Berlin, Germany
2 Johannes-Guttenberg Universität, Department of Neuropathology, Mainz, Germany
Introduction: Traditional imaging of sections in diagnostic or research settings using transmission electron microscopy (TEM) is limited by manual acquisition procedures. Thus, analysis of samples is restricted to images of few preselected regions that may be non-representative and lack correlation to their microanatomical context. Modern scanning electron microscopes allow unbiased digitization of entire large sections and improved analysis, but performance is limited by preparation artifacts.
Objectives: We aimed at an improved preparation of sections for large-scale electron microscopy (EM) and at a pipeline for data processing and analysis.
Material & methods: We achieved wrinkle-free collection of sections by a combined glow-discharge and ethanol-based smoothening procedure and reduced filming and staining artifacts. A scanning electron microscope with a scanning transmission electron microscopy detector was used for pre-irradiation and imaging. Imaging parameters were varied to achieve fast imaging and adequate image quality. We used TrakEM2 and nip2 for stitching of image tiles to generate coherent bigtif datasets, thus allowing in-depth analysis via QuPath.
Results: Our preparation workflow allowed to reliably prepare samples with virtual absence of limiting artifacts, such wrinkles and stain precipitates. Up to 12 entire sections were automatically digitized in about 6 days using 7.3 nm pixel size and 1.0 µs dwell time. Our data processing pipeline allowed batch generation of bigtif files with minimal operator involvement.
Conclusion: We propose a highly reliable workflow for preparation, imaging and data processing of samples with superior quality to enable routine large-scale EM in diagnostic and research settings.
We demonstrate the high pragmatic value of our workflow with data of different published and unpublished projects in the field of muscle pathology and ultrastructural characterization of SARS-CoV-2 in autopsy tissues.
PIII-02
Free Neuropathol 2:22:38
NanoString technology distinguishes anti-TIF-1γ+ from anti-Mi-2+ Dermatomyositis patients
C. Preuße1,2, P. Eede1, L. Heinzeling3,4, K. Freitag1,5, R. Koll1,6, W. Froehlich3, U. Schneider7, Y. Allenbach8, O. Benveniste8, A. Schänzer9, H. H. Goebel1, W. Stenzel1, J. Radke1,6
1 Charité – Universitätsmedizin Berlin, Neuropathology, Berlin, Germany
2 Münster University Hospital, Neurology with Institute for Translational Neurology, Münster, Germany
3 University Hospital of Erlangen, Dermatology, Erlangen, Germany
4 LMU Klinikum, Dermatology, München, Germany
5 German Center for Neurodegenerative Diseases (DZNE) within the Helmholtz Association, Berlin, Germany
6 German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Berlin, Germany
7 Charité – Universitätsmedizin Berlin, Rheumatology, Berlin, Germany
8 Pitié-Salpêtrière University Hospital, Internal Medicine and Clinical Immunology, Paris, France
9 Justus-Liebig-University Gießen, Neuropathology, Gießen, Germany
Introduction: Dermatomyositis (DM) is a systemic idiopathic inflammatory disease affecting skeletal muscle and skin, clinically characterized by symmetrical proximal muscle weakness and typical skin lesions. Recently, myositis-specific autoantibodies (MSA) became of utmost importance because they strongly correlate with distinct clinical manifestations and prognosis. Antibodies against transcription intermediary factor 1γ (TIF-1γ) are frequently associated with increased risk of malignancy, a specific cutaneous phenotype and limited response to therapy in adult DM patients. Anti-Mi-2 autoantibodies, in contrast, are typically associated with classic DM rashes, prominent skeletal muscle weakness, better therapeutic response and prognosis, and less frequently with cancer. The correlation of cancer and Mi-2 autoantibodies, however, remains debated.
Objectives: To further investigate the both clinically distinct DM subgroups and visualize differences or similarities on gene and protein level.
Patients & methods: We analyzed 30 DM patients (n=15 Mi2+ and n=15 TIF-1γ+) and 8 non-disease controls (NDC) by NanoString, qPCR, and immunohistochmistry.
Results: Both anti-TIF-1γ+ and anti-Mi-2+ patients" skeletal muscle biopsies revealed strong dysregulation of immune response-related genes compared to NDCs. In line with previous reports, these included well-known type 1 IFN-inducible genes; however, we also identified subgroup-specific differences.
Conclusion: Our results help to stratify both subgroups and predict, which DM patients require an intensified diagnostic procedure and might have a poorer outcome. Additionally, we demonstrate that the NanoString technology can be used as a very sensitive method to clearly differentiate two clinically distinct DM subgroups, namely Mi2+ and TIF-1γ+ Dermatomyositis. Potentially, this could also have implications for the therapeutic approach.
PIII-03
Free Neuropathol 2:22:40
Immunomodulatory function of GM-CSF in idiopathic inflammatory myopathies
D. Cengiz1,2, T. Müntefering2, J. Schubert1, C. Preuße3, W. Stenzel3, S. Meuth2, T. Ruck2
1 Clinic for Neurology with Institute for Translational Neurology, University Hospital, Münster, Germany
2 Heinrich-Heine University Düsseldorf, Department of Neurology, Medical Faculty, Düsseldorf, Germany
3 Charité Universitätsmedizin, Department of Neuropathology, Berlin, Germany
Introduction: Idiopathic inflammatory myopathies (IIMs) are characterized by chronic inflammation of the muscle, resulting in muscle weakness and pain. Pathogenesis is driven by the interaction of skeletal muscle, muscle endothelial and immune cells. Cytokines play a central role in controlling these interactions. GM-CSF is a cytokine, which is significantly involved in the development of autoimmune diseases and is currently being investigated in clinical trials for therapy.
Objectives: The role of GM-CSF in the inflammatory processes of IIMs is largely unknown. Therefore, the aim of this work is to investigate its immunomodulatory function.
Materials & Methods: Murine skeletal muscle and endothelial cells were stimulated with GM-CSF and other pro- and anti-inflammatory cytokines. To analyze the impact of these conditions, flow cytometric analysis was performed. Furthermore, the immunological relevance of GM-CSF on immune cell migration and adhesion were investigated. The expression of GM-CSF was also evaluated in human muscle biopsies of IIM patients.
Results: Stimulation with pro- and anti-inflammatory cytokines regulate the expression of GM-CSF and the GM-CSF receptor in muscle and endothelial cells. GM-CSF led to increased expression of costimulatory molecules on skeletal muscle cells, as well as decreased expression of adhesion molecules on endothelial cells. Stimulation also resulted in increased immune cell migration across muscle endothelial barriers. In human muscle biopsies of IIM patients, histological analysis revealed high expression of GM-CSF.
Conclusion: The GM-CSF signaling pathway affects the cellular interactions of muscle, endothelial, and immune cells in the skeletal muscle. These findings could contribute to elucidate autoimmune processes in IIMs and identify new therapeutic targets.
PIII-04
Free Neuropathol 2:22:41
Effect of vasoactive therapy in patients with Systemic Sclerosis on dermal small nerve fibers, Langerhans-cells and vessel density.
L. Bajors1, F. Höcketstaller2, M. Köhm2,3, S. Seidel4, R. Wolf5, A. Roth1, U. Drott2,6, A. Schänzer1
1 Justus-Liebig-University Gießen, Institute of Neuropathology, Gießen, Germany
2 Goethe University Frankfurt, Department of Rheumatology, Frankfurt a. M., Germany
3 Goethe University Frankfurt, Fraunhofer Project Group Translational Medicine and Pharmacology, IME, Frankfurt a. M., Germany
4 Goethe University Frankfurt, Institute of Cell Biology and Neuroscience and Buchmann Institute for Molecular Life Sciences (BMLS), Frankfurt a. M., Germany
5 Philipps University Marburg, Department of Dermatology and Allergology, Marburg, Germany
6 MEDIAN Klinik Schlangenbad, Department of Rheumatology, Wiesbaden, Germany
Introduction: Systemic Sclerosis (SSc) is an orphan immune-mediated disease affecting multiple organ systems. Most commonly, clinical symptoms such as skin fibrosis and digital ulceration occur early in disease course. Here, vasculopathy is an underlying aetiology. Additional, small nerve fibers (SNF) might be affected in in SSc patients and contribute to disease progression.
Material and Methods: 21 SSc patients received intravenous medication iloprost for 10 days (95% females; 56,1±11,9 years). 7 healthy subjects served as controls (71% f; 51,6±18,3 y). Skin biopsies were taken from the volar forearm before and 3 months after treatment. Epidermal nerve density (END), Langerhans cells (LC) and vessel density (VD) were analysed at immunofluorescence-stained sections using a Zeiss AxioScanner and imageJ software. The skin pathology was categorized with a histological skin score.
Results: The SSc group showed no significant difference of END and LC density (13,09±4,26 SNF/mm; 259,8±121,5 LC/mm²) compared to controls (10,8±3,11 SFN/mm, p=0,198; 335,4±124,86 LC/mm², p=0,091) or after treatment (12,71±5,82 SNF/mm, p=0,49; 305,4±98,5 LC/mm², p=0,098). The VD was significantly increased in the SSc group compared to controls (3,01±1,10µm²/100µm²; 2,18±1,12µm²/100µm²; p=0,046). In the SSc group, the VD was significantly decreased after treatment (2,51±0,78µm²/100µm²; p=0,044). The histological skin score showed overall low fibrotic alterations in both groups.
Conclusion: In our study, SSc patients showed a higher VD compared to controls and VD was affected during short-term iloprost-therapy. Megacapillaries is a key feature of SSc and the results should be correlated with results of capillary microscopy. END and LC were not altered during treatment in SSC patients. Maybe these findings depend on the low skin score showed in the patients. To investigate further correlations, the results will be correlated with clinical parameters.
Poster session IV – Neurodegeneration
PIV-01
Free Neuropathol 2:22:42
The interplay between neuroimmune activation and blood brain barrier dysfunction in Alzheimer's disease
M. Armbrust1, A. Dominguez-Belloso1, R. Figueiredo2, K. Gneiding1, S. Guérit1, S. Thom1, P. Winter2, K. H. Plate1, P. N. Harter1, K. Devraj1, S. Liebner1
1 Goethe University Hospital, Neurological Institute, Edinger Institute, Frankfurt a. M., Germany
2 GenXPro GmbH, Frankfurt a. M., Germany
Introduction: Alzheimer's disease (AD) is characterized by amyloid-β aggregation, hyperphosphorylated tau protein, neuroimmune activation, neuronal loss and blood brain barrier (BBB) dysfunction. The contribution of the latter to disease genesis and progression is poorly understood.
Objectives: To investigate Wnt/β-catenin and BBB-specific alterations in AD.
Materials and Methods: Transgenic mice harbouring triple-mutated (SwDI) human APP were used for 3'-RNA-sequencing (Massive Analysis of cDNA Ends: MACE) of isolated mouse brain microvessels (MBMVs) and of FACS-sorted cells of the neurovascular unit (endothelial cells (ECs), mural cells, astrocytes and microglia (MG)). An activation of the Wnt/β-catenin pathway in ECs was conducted in a Cre-LoxP-based β-catenin gain-of-function (GOF)-AD mouse model. Tracer permeability assays, immunohistochemistry and immunofluorescence analyses as well as behavourial tests were performed in AD and AD-GOF mice.
Results: Gene Ontology analysis revealed a downregulation of Wnt/β-catenin signalling in MBMVs and FACS-sorted ECs of AD mice. Dkk2, encoding Dickkopf Wnt pathway inhibitor 2, was upregulated in MBMVs and MG. In human AD brain tissue, DKK2 was detected in MG. Additionally, an increase in BBB permeability correlating with cognitive decline and a decrease of vascular lymphoid enhancer-binding factor 1 expression, a target and key player in Wnt/β-catenin signalling, was observed in AD mice. A presymptomatic activation of the Wnt/β-catenin pathway in ECs of GOF-AD mice partially prevented cognitive decline.
Conclusion: DKK2 upregulation in microvessel-associated MG could contribute to the Wnt/β-catenin pathway repression observed at the BBB in AD. Together with the prevention of cognitive decline after presymptomatic activation of the Wnt/β-catenin pathway, our findings indicate a potential link between neuroimmune activation and BBB dysfunction and propose the Wnt/β-catenin pathway as a potential therapeutic target.
PIV-02
A deep learning approach for monitoring parietal-dominant Alzheimer's disease in World Trade Center responders at midlife
not available for publication
PIV-03
Free Neuropathol 2:22:44
Amyloid-β dimers (Aβ-S8C) are antiprions inhibiting seeded nucleation in vivo
E. van Gerresheim1, A. Müller-Schiffmann1, S. Schäble2, C. Korth1
1 Heinrich-Heine University Düsseldorf, Neuropathology, Düsseldorf, Germany
2 Heinrich-Heine University Düsseldorf, Experimental Psychology, Düsseldorf, Germany
Introduction: Alzheimer"s disease (AD) is a neurologic disorder, were amyloid-beta (Aβ) aggregation causes massive neuronal death. Aβ expansion can follow prion-like replication from Aβ seeds. With the current animal models, based on the overexpression of Aβ, used to study the Aβ cascade and prion-like expansion, it is impossible to distinguish between exogenous de novo seeding and accelerated spontaneous plaque development. So far, seeding of Aβ in wild type animals has not been successful.
Objectives: Here we studied the effects of Aβ-S8C dimers on Aβ prion spreading and induced behavioral changes.
Materials and Methods: TgDimer mice, expressing human APP751 (Swe, K670 N/ M671L, S679C), only develop Aβ-S8C dimers and do not develop Aβ plaques, but do associate to existing plaques, were crossed with Gfap-luc mice, enabling bioluminescence (BLI) monitoring of Aβ aggregation-associated astrogliosis. Mice were intra-hippocampal inoculated with Aβ aggregates. Wild type and APP23 mice, Aβ aggregate inoculated, were used as controls. Cognition was probed in the observer-free paradigm, the CognitionWall, for reward-dependent learning and burrowing as species-specific behavior.
Results: We observed no Aβ aggregation-associated astrogliosis or impaired cognition in the tgDimer mice inoculated with Aβ aggregate. For the positive control, the APP23 mice, an increase in BLI signal started at 11 months of age and at 18 months of age, APP23 mice showed impaired discrimination learning in the CognitionWall.
Conclusions: Aβ-S8C dimers inhibit Aβ aggregate spreading and are thus anti-prions. They may have a regulatory role for aggregate spreading suggesting that Aβ prion effects are actively regulated rather than a passive process. The difference in time of start of Aβ aggregate spreading and impaired discrimination learning in APP23 mice suggests that behavioral consequences are disparate from initial neuropathological signs.
PIV-04
Free Neuropathol 2:22:45
A decline in peroxisomal numerical abundance occurs late during Alzheimer´s disease progression and is particulary found in the hippocampus
E. Semikasev1, B. Ahlemeyer1, T. Acker2, A. Schänzer2, E. Baumgart-Vogt1
1 Institute of Anatomy and Cell Biology, Medical Cell Biology, JL University, Gießen, Germany
2 Institute of Neuropathology, JL University, Gießen, Germany
Peroxisomal biogenesis disorders are inherited metabolic diseases caused by mutations in the PEX genes. In severe forms, patients have profound defects in the brain demonstrating the importance of this organelle for neuronal survival and function. Additionally, dysfunction of the peroxisomal metabolism, e.g. plasmalogen synthesis or the degradation of very long-chained fatty acids, has to been linked to neurodegenerative diseases such as Alzheimer´s disease (AD).
To study the role of peroxisomes in AD, we analyzed the density of this organelle in the perikarya of pyramidal cells in the hippocampus (cornu ammonis, subiculum and the entorhinal cortex layer III) as well as neocortical regions (frontal, temporal, parietal und occipital) of brain from patients with different neuropathological stages.
We used double immunofluorescence staining techniques to detect PEX14 (as marker for the total peroxisome numerical abundance) and β-amlyoid plaques or neurofibrillary tangles (NFTs)s in different brain regions. Human brains were gender- and age-matched and classified into 5 patient groups based to the ABC-Score. All 42 brain samples were from the Institute of Neuropathology in Gießen. Quantification of peroxisome number per area (density) was performed by ImageJ free software.
With ongoing stages of AD, we measured a progressive decline in the density of peroxisomes in pyramidal cells of all hippocampal and neocortical areas. The effect was even more pronounced when we included known AD risk factors such as vascular angiopathies. Interestingly, the decrease in the density of peroxisomes at late stages in AD was more evident in patients ≤80 years old than in patients >81 years old. However, we did not find a correlation between the intraneuronal peroxisome density and the amount of β-amyloid and NFTs.
Our data suggest that peroxisomes may play a protective role in AD and their decline in numerical density – especially in the hippocampus – occurs late during AD progression.
PIV-05
Free Neuropathol 2:22:46
Its not all about Covid19 - Incidental finding of hereditary Neuroferritinopathy in a 78-year-old woman diagnosed with COVID-19 associated pneumonia
V. Umathum1,2, D. Amsel1, A. Weber3, C. Becker4, C. Kasan4, A. May5, T. Acker1, A. Schänzer1
1 Justus-Liebig-University Gießen, Institute of Neuropathology, Gießen, Germany
2 Bundeswehrkrankenhaus Ulm, Institute of Pathology and Molecular Pathology, Ulm, Germany
3 Justus-Liebig-University Gießen, Institute of Human Genetics, Gießen, Germany
4 Institute of Pathology, Cytology and Molecular Pathology, Wetzlar, Germany
5 Agaplesion Evangelisches Krankenhaus, Department of Pulmonology, Gießen, Germany
Introduction: Neuroferritinopathy (NF) is an autosomal-dominant movement disorder due to mutations in the L-ferritin gene (FTL) on chromosome 19q13.33. NF belongs to neurodegeneration with brain iron accumulation (NBIA) disorders, a group of hereditary neurodegenerative diseases with a prevalence of 1/1.000.000. Patients with NF present clinically with progressive extrapyramidal movement symptoms with variable phenotype. Histopathologically, NF is characterized by iron deposits and formation of ferritin inclusion bodies (IBs).
Clinical History: A 78-year-old woman was hospitalized with a severe Covid-19 pneumonia and after three months died from Covid19-associated pneumonia. During her 3-month clinical course she showed neurological symptoms of delirium and restlessness. Cranial MRI was not performed.
Neuropathological Findings: Brain weight was 1172 g. No abnormalities were seen in cross sections. Histological examination revealed frequent atypical iron accumulation throughout the brain particularly in the basal ganglia. The nuclear spherical iron inclusions were localized in neurons, microglia and oligodendrocytes stained for Prussian blue and immunohistochemically with antibodies against Anti-Ferritin Light Chain. Electron microscopy showed fragmented nuclei with inclusions and chromatin accumulation toward the membrane consistent with IBs. Moderate microcystic vacuolization and partial loss of myelination of nerve fibres were evident.
Discussion and Conclusion:131 cases of NF have been described so far in the literature with 80% in France and the UK and 20% throughout the world. Movement disorders or cognitive impairments were not reported in our patient, maybe due to a lack of full penetrance that occurs at the age of 60, in general. In our patient, the brain pathology is suspicious for a NF. Most of the patients harbour an adenine insertion at position 460-461 of the FTL-Gene. Whole exome sequencing from brain tissue is in progress to confirm the diagnosis.
PIV-06
Free Neuropathol 2:22:47
Role of purinergic receptor P2Y12 in Alzheimer's disease
R. Maruccia1,2, A. A. Gabr1, M. J. Pietrowski1, B. A. Tambe1, A. Halle1,2
1 Deutsches Zentrum für Neurodegenerative Erkrankungen, Bonn, Germany
2 Universitätsklinikum Bonn, Institut für Neuropathologie, Bonn, Germany
Microglia have recently moved into prime focus of AD research due to the discovery of risk loci and genes that are linked to immune mechanisms, some of them highly expressed in microglia. However, immune signaling pathways in microglia that modulate AD progression, for example by removing Aβ plaques, have remained poorly understood.
The purinergic receptor P2Y12 is activated by extracellular ATP and ADP and is highly expressed in platelets and microglia. P2Y12 is crucial for several microglial functions such as chemotaxis, blood-brain barrier closure, synaptic plasticity, interaction with neuronal somata and phagocytosis. Thus, a compromised microglial phagocytic removal of Aβ and impaired plaque barrier formation upon P2Y12 deletion may affect AD progression.
Here we analyze 3D-reconstructed confocal images of brain tissue from AD models to assess the role of P2Y12 in the progression of Aβ pathology, microglia-plaque interaction and inter-cellular interaction between microglial cells under neurodegenerative conditions.
Our preliminary findings suggest a region-dependent modulation of microglial coverage and co-localization with plaques in models with P2Y12 deletion and therefore that this receptor may affect microglial interaction with Aβ plaques.
Future studies investigating plaque load changes and the interplay between microglia, astrocytes and neurons will be needed to better understand inter-cellular pathways that contribute to P2Y12-related effects in AD pathology.
Copyright: © 2021 The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited, a link to the Creative Commons license is provided, and any changes are indicated. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
|